Abstract
Introduction
Decreased fractional anisotropy (FA) demonstrated by diffusion tensor MR imaging (DTI) in areas of white matter (WM) damage is generally associated with increase of radial diffusivity, while axial diffusivity is reported to be decreased, unchanged, or increased. Aiming to better define the type of axial diffusivity change occurring in a typical human neurodegenerative disease, we investigated axial and radial diffusivity in Friedreich's ataxia (FRDA) which is characterized by selective neuronal loss of the dentate nuclei and atrophy and decreased FA of the superior cerebellar peduncles (SCPs).
Methods
Axial and radial diffusivity of the whole-brain WM were evaluated in 14 patients with FRDA and 14 healthy volunteers using DTI at 1.5 T and the tract-based spatial statistics (TBSS) method, part of FSL software.
Results
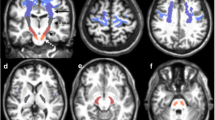
TBSS analysis showed a single area in the central midbrain corresponding to the decussation of the SCPs which exhibited lower FA in patients than in controls. In this area, a significant increase of both axial and radial diffusivity was observed. No clusters of significantly decreased axial diffusivity were observed, while additional clusters of increase of radial diffusivity were present throughout the brain.
Conclusions
The selective decrease of FA in SCPs of FRDA patients reflecting chronic WM tract damage is associated with increase of both the axial and radial diffusivity, the latter more pronounced than the former. The ultrastructural and biophysical bases of the increased axial diffusivity in chronically degenerating WM tracts deserve further studies.



Similar content being viewed by others
References
Basser PJ, Pierpaoli C (1996) Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Reson B 111:209–219
Song SK, Sun SW, Ju WK et al (2003) Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. NeuroImage 20:1714–1722
Budde MD, Kim JH, Liang HF et al (2007) Toward accurate diagnosis of white matter pathology using diffusion tensor imaging. Magn Reson Med 57:688–695
Sun SW, Liang HF, Cross AH et al (2008) Evolving Wallerian degeneration after transient retinal ischemia in mice characterized by diffusion tensor imaging. NeuroImage 40:1–10
Budde MD, Xie M, Cross AH et al (2009) Axial diffusivity is the primary correlate of axonal injury in the EAE spinal cord: a quantitative pixelwise analysis. J Neurosci 29:2805–2813
Kim JH, Loy DN, Wang Q et al (2010) Diffusion tensor imaging at 3 hours after traumatic spinal cord injury predicts long-term locomotory recovery. J Neurotrauma 27:587–598
Concha L, Gross DW, Wheatley M et al (2006) Diffusion tensor imaging of time dependent axonal and myelin degradation after corpus callosotomy in epilepsy patients. NeuroImage 32:1090–1099
Lowe J, Lennox G, Leigh PN (1997) Disorders of movement and system degeneration. In: Graham DL, Lantos PL (eds) Greenfield's neuropathology, vol. 2, 6th edn. Arnold, London, pp 281–366
Cosottini M, Giannelli M, Siciliano G et al (2005) Diffusion-tensor MR imaging of corticospinal tract in amyotrophic lateral sclerosis and progressive muscular atrophy. Radiology 237:258–264
Rosas HD, Lee SY, Bender AC et al (2010) Altered white matter microstructure in the corpus callosum in Huntington's disease: implications for cortical “disconnection”. NeuroImage 49:2995–3004
Weaver KE, Richards TL, Liang O et al (2009) Longitudinal diffusion tensor imaging in Huntington's disease. Exp Neurol 216:525–529
Acosta-Cabronero J, Williams GB, Pengas G et al (2010) Absolute diffusivities define the landscape of white matter degeneration in Alzheimer's disease. Brain 133:529–539
Salat DH, Tuch DS, van der Kouwe AJ et al (2010) White matter pathology isolates the hippocampal formation in Alzheimer's disease. Neurobiol Aging 31:255–256
Della Nave R, Ginestroni A, Tessa C et al (2010) Regional distribution and clinical correlates of white matter microstructural damage in Huntington's disease: a tract-based spatial statistics study. AJNR Am J Neuroradiol 31:1675–1681
Della Nave R, Ginestroni A, Tessa C et al (2008) Brain white matter tracts degeneration in Friedreich ataxia. An in vivo MRI study using tract-based spatial statistics and voxel based morphometry. NeuroImage 40:19–25
Smith SM, Jenkinson M, Johansen-Berg H et al (2006) Tract based spatial statistics: voxelwise analysis of multi-subject diffusion data. NeuroImage 31:1487–1505
Bidichandani SI, Ashizawa T, Patel PI (1998) The GAA triplet-repeat expansion in Friedreich ataxia interferes with transcription and may be associated with an unusual DNA structure. Am J Hum Genet 62:111–121
Filla A, De Michele G, Caruso G et al (1990) Genetic data and natural history of Friedreich's disease: a study of 80 Italian patients. J Neurol 237:345–351
Smith SM, Jenkinson M, Woolrich MW et al (2004) Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage 23:208–219
Nichols TE, Holmes AP (2002) Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum Brain Mapp 15:1–25
Wakana S, Jiang H, Nagae-Poetscher LM et al (2004) Fiber tract-based atlas of human white matter anatomy. Radiology 230:77–87
Testut L, Latarjet A (1971) Traité d'anatomie humaine, 9th edn. G. Dion & C.ie, Paris
Sullivan EV, Rohlfing T, Pfefferbaum A (2010) Quantitative fiber tracking of lateral and interhemispheric white matter systems in normal aging: relations to timed performance. Neurobiol Aging 31:464–481
Waldvogel D, van Gelderen P, Hallett M (1999) Increased iron in the dentate nucleus of patients with Friedrich's ataxia. Ann Neurol 46:123–125
Ciccarelli O, Catani M, Johansen-Berg H et al (2008) Diffusion-based tractography in neurological disorders: concepts, applications, and future developments. Lancet Neurol 7:715–727
Rewcastle NB (1991) Degenerative diseases of the central nervous system. In: Davis RL, Robertson DM (eds) Textbook of neuropathology, 2nd edn. Williams and Wilkins, Baltimore, pp 904–906
Beaulieu C (2002) The basis of anisotropic water diffusion in the nervous system—a technical review. NMR Biomed 15:435–455
Pagani E, Ginestroni A, Salvi F et al (2010) Assessment of brain white matter fibre bundle atrophy in patients with Friedreich's ataxia. Radiology 255:882–888
Englund E, Sjobeck M, Brockstedt S et al (2004) Diffusion tensor MRI post mortem demonstrated cerebral white matter pathology. J Neurol 251:350–352
Della Nave R, Ginestroni A, Giannelli M et al (2008) Brain structural damage in Friedreich ataxia. J Neurol Neurosurg Psychiatry 79:82–85
Iltis I, Hutter D, Bushara KO et al (2010) 1H MR spectroscopy in Friedreich's ataxia and ataxia with oculomotor apraxia type 2. Brain Res 1358:200–210
Smith SM, Johansen-Berg H, Jenkinson M et al (2007) Acquisition and voxelwise analysis of multi-subject diffusion data with tract-based spatial statistics. Nat Protoc 3:499–503
Conflict of Interest
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Fig. 1
TBSS analysis of whole-brain λ|| map in FRDA patients vs. healthy controls. Map of the t value (t > 3 with p < 0.05 corrected for multiple comparisons) showing in violet the areas of significantly increased λ|| that are solely located in the central midbrain corresponding to the SCP decussation (GIF 54 kb)
Supplementary Fig. 2
TBSS analysis of whole-brain λ⊥ map in FRDA patients vs. healthy controls. Map of the t value (t > 3 with p < 0.05 corrected for multiple comparisons) showing in sky-blue the areas of significantly increased λ⊥ that are located in the central midbrain, corresponding to the SCP decussation, the SCPs, the subcortical cerebellar WM, the inferior fronto-occipital fasciculi and the corticospinal tracts, bilaterally, the right inferior longitudinal fasciculus and the left frontal subcortical WM (GIF 76 kb)
Rights and permissions
About this article
Cite this article
Della Nave, R., Ginestroni, A., Diciotti, S. et al. Axial diffusivity is increased in the degenerating superior cerebellar peduncles of Friedreich's ataxia. Neuroradiology 53, 367–372 (2011). https://doi.org/10.1007/s00234-010-0807-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-010-0807-1