Abstract
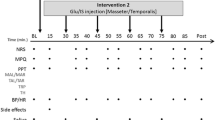
Temporomandibular disorders (TMDs) are associated with perturbation of proprioceptive and nociceptive function. Recent studies have shown that injection of the neurotrophic protein nerve growth factor (NGF) into the masseter muscle causes sensitization to mechanical pressure stimuli; however, it is not clear if vibration sense and jaw stretch reflexes as measures of proprioceptive function as well as glutamate-evoked pain are also altered. We tested the hypothesis that NGF-induced mechanical sensitization would be associated with changes in vibration sense and stretch reflex sensitivity as well as facilitation of glutamate-evoked pain responses. A double-blind, randomized and placebo-controlled study was conducted on 14 healthy men. In one session subjects received an injection of NGF (5 μg in 0.2 ml) into the masseter muscle and in a control session an injection of buffered isotonic saline (0.9%, 0.2 ml). Subjects assessed their pain intensity on a 0–10 cm visual analogue scale (VAS) for 15 min after the injections. Pressure pain thresholds (PPT), vibration sense and jaw stretch reflexes were recorded at baseline and 1, 2, 3 and 24 h post-injection. The sensitivity to injections of glutamate into the masseter muscle (1 M, 0.2 ml) was assessed after 24 h. ANOVAs were used to assess significant differences. NGF did not cause more pain than isotonic saline, but significantly reduced PPTs 1, 2, 3 and 24 h post-injection (P < 0.001) whereas isotonic saline had no effects on PPTs (P = 0.583). The injection of glutamate after 24 h was associated with reduced PPTs in both sessions, but the PPTs remained lower in the NGF pretreated masseter than in the control masseter (P < 0.001). Ratings of vibratory stimuli and the normalized amplitude of the jaw stretch reflex were not affected by the NGF-induced sensitization; however, after glutamate injection a significant increase in the stretch reflex was observed in the injected masseter muscle in both sessions (P = 0.002). There were no significant differences in the perceived pain intensity of the glutamate injection between the masseter muscle pretreated with NGF or control (P > 0.414), although the glutamate-evoked pain drawing areas were larger for the NGF-pretreated masseter muscle (P = 0.009). In conclusion, this study confirms that masseter muscle injection of NGF is associated with a distinct and prolonged sensitization to mechanical stimuli, but without an effect on large-diameter mechanoreceptive and the muscle spindle afferents. Additional challenge of the NGF pretreated muscle with glutamate did not indicate a conspicuous sensitization to noxious chemical stimuli. These findings are discussed in terms of the concept of “proprioceptive allodynia”.





Similar content being viewed by others
References
Arima T, Svensson P, Arendt-Nielsen L (2000) Capsaicin-induced muscle hyperalgesia in the exercised and non-exercised human masseter muscle. J Orofac Pain 14:213–223
Biasiotta A, Peddireddi A, Wang K, Romaniello A, Svensson P, Arendt-Nielsen L (2007) Effect of pinching-evoked pain on jaw-stretch reflexes and exteroceptive suppression periods in healthy subjects. Clin Neurophysiol 118:2180–2188
Cairns BE, Hu JW, Arendt-Nielsen L, Sessle BJ, Svensson P (2001) Sex-related differences in human pain perception and rat afferent discharge evoked by injection of glutamate into the masseter muscle. J Neurophysiol 86:782–791
Cairns BE, Wang K, Hu JW, Sessle BJ, Arendt-Nielsen L, Svensson P (2003a) The effects of glutamate-evoked masseter muscle pain on the human jaw-stretch reflex differs in men and women. J Orofac Pain 17:317–325
Cairns BE, Svensson P, Wang K, Hupfeld S, Graven-Nielsen T, Sessle BJ, Berde CB, Arendt-Nielsen L (2003b) Activation of peripheral NMDA receptors contributes to human pain and rat afferent fiber discharge evoked by injection of glutamate into the masseter muscle. J Neurophysiol 90:2098–2105
Cairns BE, Svensson P, Wang K, Castrillon E, Hupfeld S, Sessle BJ, Arendt-Nielsen L (2006) Ketamine attenuates glutamate-induced mechanical sensitization of the masseter muscle in human males. Exp Brain Res 169:467–472
Capra NF, Hisley CK, Masri RM (2007) The influence of pain on masseter spindle afferent discharge. Arch Oral Biol 52:387–390
Castrillon E, Cairns BE, Ernberg M, Wang K, Sessle BJ, Arendt-Nielsen L, Svensson P (2007) Effect of a peripheral NMDA receptor antagonist on glutamate-evoked masseter muscle pain and mechanical sensitization in women. J Orofac Pain 21:216–224
Chao MV, Hempstead BL (1995) p75 and Trk: a two-receptor system. Trends Neurosci 18:321–326
Chao MV (2003) Neurotrophins and their receptors: A convergence point for many signaling pathways. Neuroscience 4:299–309
Di Luca M, Gardoni F, Finardi A, Pagliardini S, Cattabeni F, Battaglia G, Missale C (2001) NMDA receptor subunits are phophorylated by activation of neurotrophin receptors in PSD of rat spinal cord. Neuroreport 12:1301–1305
Dong XD, Mann MK, Kumar U, Svensson P, Arendt-Nielsen L, Hu JW, Sessle BJ, Cairns BE (2007) Sex-related differences in NMDA-evoked rat masseter muscle afferent discharge result from estrogen-mediated modulation of peripheral NMDA receptor activity. Neuroscience 146:822–832
Drewes AM, Helweg-Larsen S, Petersen P, Brennum J, Andreasen A, Poulsen LH, Jensen TS (1993) McGill Pain Questionnaire translated into Danish: experimental and clinical findings. Clin J Pain 9:80–87
Dyck PJ, Peroutka S, Rask C, Burton E, Baker MK, Lehman KA, Gillen DA, Hokanson JL, O’Brien PC (1997) Intradermal recombinant human nerve growth factor induces pressure allodynia and lowered heat-pain threshold in humans. Neurology 48:501–505
Dworkin SF, LeResche L (1992) Research diagnostic criteria for temporomandibular disorders: review, criteria, examinations and specifications, critique. J Craniomandib Disord Facial Oral Pain 6:301–355
Fillingim RB, Fillingim LA, Hollins M, Sigurdsson A, Maixner W (1998) Generalized vibrotactile allodynia in a patient with temporomandibular disorder. Pain 78:75–78
Hoheisel U, Unger T, Mense S (2005) Excitatory and modulatory effects of inflammatory cytokines and neurotrophins on mechanosensitive group IV muscle afferents in the rat. Pain 114:168–176
Hollins M, Sigurdsson A (1998) Vibrotactile amplitude and frequency discrimination in temporomandibular disorders. Pain 75:59–67
Hollins M, Sigurdsson A, Fillingim L, Goble AK (1996) Vibrotactile threshold is elevated in temporomandibular disorders. Pain 67:89–96
Hollins M, Sigurdsson A, Morris KA (2001) Local vibrotactile and pain sensitivities are negatively related in temporomandibular disorders. J Pain 2:46–56
Jarvis CR, Xiong ZG, Plant JR, Churchill D, Lu WY, MacVicar BA, MacDonald JF (1997) Neurotrophin modulation of NMDA receptors in cultured murine and isolated rat neurons. J Neurophysiol 78:2363–2371
Koh S, Oyler GA, Higgins GA (1989) Localization of nerve growth factor receptor messenger RNA and protein in the adult rat brain. Exp Neurol 106:209–221
Kurose M, Yamamura K, Noguchi M, Inoue M, Ootaki S, Yamada Y (2005) Modulation of jaw reflexes induced by noxious stimulation to the muscle in anesthetized rats. Brain Res 1041:72–86
Makowska A, Panfil C, Ellrich J (2005) Nerve growth factor injection into semispinal neck muscle evokes sustained facilitation of the jaw-opening reflex in anesthetized mice–possible implications for tension-type headache. Exp Neurol 191:301–309
Mann MK, Dong X-D, Svensson P, Cairns BE (2006) Influence of intramuscular nerve growth factor injection on the response properties of rat masseter muscle afferent fibers. J Orofac Pain 20:325–336
McMahon SB, Bennett DLH, Bevan S (2006) Inflammatory mediators and modulators of pain. In: McMahon SB, Koltzenburg M (eds) Textbook of pain. Elsevier Churchill Livingstone, New York, pp 49–72
Melzack R (1975) The McGill Pain Questionnaire: major properties and scoring methods. Pain 1:277–299
Michael GJ, Averill S, Nitkunan A, Rattray M, Bennett DL, Yan Q, Priestley JV (1997) Nerve growth factor treatment increases brain-derived neurotrophic factor selectively in TrkA-expressing dorsal root ganglion cells and in their central terminations within the spinal cord. J Neurosci 17:8476–8490
Miles TS, Poliakov AV, Flavel SC (1993) An apparatus for controlled stretch of human jaw-closing muscles. J Neurosci Methods 46:197–202
Peddireddy A, Wang K, Svensson P, Arendt-Nielsen L (2005) Effect of experimental posterior temporalis muscle pain on human brainstem reflexes. Clin Neurophysiol 116:1611–1620
Petty BG, Cornblath DR, Adornato BT, Chaudhry V, Flexner C, Wachsman M, Sinicropi D, Burton LE, Peroutka SJ (1994) The effect of systemically administered recombinant human nerve growth factor in healthy human subjects. Ann Neurol 36:244–246
Pezet S, McMahon SB (2006) Neurotrophins: mediators and modulators of pain. Annu Rev Neuroscience 29:507–538
Sarchielli P, Alberti A, Floridi A, Gallai V (2001) Levels of nerve growth factor in cerebrospinal fluid of chronic daily headache patients. Neurology 57:132–134
Sarchielli P, Alberti A, Candeliere A, Floridi A, Capocchi G, Calabresi P (2006) Glial cell line-derived neurotrophic factor and somatostatin levels in cerebrospinal fluid of patients affected by chronic migraine and fibromyalgia. Cephalalgia 26:409–415
Sarchielli P, Mancini ML, Floridi A, Coppola F, Rossi C, Nardi K, Acciarresi M, Alberto Pini L, Calabresi P (2007) Increased levels of neurotrophins are not specific for chronic migraine: evidence from primary fibromyalgia syndrome. J Pain 8:737–745
Svensson P, Arendt-Nielsen L (1996) Effects of 5 days of repeated submaximal clenching on masticatory muscle pain and tenderness: an experimental study. J Orofac Pain 10:330–338
Svensson P, Arendt-Nielsen L, Nielsen H, Larsen JK (1995) Effect of chronic and experimental jaw muscle pain on pain-pressure thresholds and stimulus–response curves. J Orofac Pain 9:347–356
Svensson P, Cairns BE, Wang K, Arendt-Nielsen L (2003a) Injection of nerve growth factor into human masseter muscle evokes long-lasting mechanical allodynia and hyperalgesia. Pain 104:241–247
Svensson P, Cairns BE, Wang K, Hu JW, Graven-Nielsen T, Arendt-Nielsen L, Sessle BJ (2003b) Glutamate-induced sensitization of pressure pain thresholds in human masseter muscle. Pain 101:221–227
Svensson P, Macaluso GM, De Laat A, Wang K (2001) Effects of local and remote muscle pain on human jaw reflexes evoked by fast stretches at different clenching levels. Exp Brain Res 139:495–502
Svensson P, Wang M, Dong X, Kumar U, Cairns B (2008) Human nerve growth factor (NGF) sensitizes masseter muscle nociceptors. Proceedings of the 12th world congress on pain, IASP Press, Seattle (in press)
Treede R-D, Klein T, Magerl W (2006) Pain memory and central sensitization in humans. In: Flor H, Kalso E, Dostrovsky JO (eds) Proceedings of the 11th world congress on pain. IASP Press, Seattle, pp 251–267
van Selms MK, Wang K, Lobbezoo F, Svensson P, Arendt-Nielsen L, Naeije M (2005) Effects of masticatory muscle fatigue without and with experimental pain on jaw-stretch reflexes in healthy men and women. Clin Neurophysiol 116:1415–1423
Wang K, Svensson P, Arendt-Nielsen L (2000) Effect of tonic muscle pain on short-latency jaw-stretch reflexes in humans. Pain 88:189–197
Wang K, Arendt-Nielsen L, Svensson P (2002) Capsaicin-induced muscle pain alters the excitability of the human jaw-stretch reflex. J Dent Res 81:650–654
Wang K, Sessle BJ, Svensson P, Arendt-Nielsen L (2004) Glutamate evoked neck and jaw muscle pain facilitate the human jaw stretch reflex. Clin Neurophysiol 115:1288–1295
Weerakkody NS, Whitehead NP, Canny BJ, Gregory JE, Proske U (2001) Large-fiber mechanoreceptors contribute to muscle soreness after eccentric exercise. J Pain 2:209–219
Weerakkody NS, Percival P, Hickey MW, Morgan DL, Gregory JE, Canny BJ, Proske U (2003) Effects of local pressure and vibration on muscle pain from eccentric exercise and hypertonic saline. Pain 105:425–435
Acknowledgments
The International Association for the Study of Pain kindly awarded a travel grant to BEC and PS. The support from the Danish National Research Foundation and Danish Dental Association is greatly appreciated.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Svensson, P., Wang, K., Arendt-Nielsen, L. et al. Effects of NGF-induced muscle sensitization on proprioception and nociception. Exp Brain Res 189, 1–10 (2008). https://doi.org/10.1007/s00221-008-1399-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-008-1399-4