Abstract
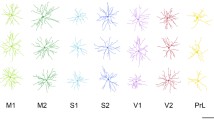
Cortical pyramidal cells, while having a characteristic morphology, show marked phenotypic variation in primates. Differences have been reported in their size, branching structure and spine density between cortical areas. In particular, there is a systematic increase in the complexity of the structure of pyramidal cells with anterior progression through occipito-temporal cortical visual areas. These differences reflect area-specific specializations in cortical circuitry, which are believed to be important for visual processing. However, it remains unknown as to whether these regional specializations in pyramidal cell structure are restricted to primates. Here we investigated pyramidal cell structure in the visual cortex of the tree shrew, including the primary (V1), second (V2) and temporal dorsal (TD) areas. As in primates, there was a trend for more complex branching structure with anterior progression through visual areas in the tree shrew. However, contrary to the trend reported in primates, cells in the tree shrew tended to become smaller with anterior progression through V1, V2 and TD. In addition, pyramidal cells in V1 of the tree shrew are more than twice as spinous as those in primates. These data suggest that variables that shape the structure of adult cortical pyramidal cells differ among species.






Similar content being viewed by others
References
Blasdel GG, Fitzpatrick D (1984) Physiological organization of layer IV in macaque striate cortex. J Neurosci 4:880–895
Bosking WH, Zhang Y, Schofield B, Fitzpatrick D (1997) Orientation selectivity and the arrangement of horizontal connections in tree shrew striate cortex. J Neurosci 17:2112–2127
Campbell CBG (1980) The nervous system of the tupaiidae: its bearing on phylogenetic relationships. Plenum, New York
Casagrande VA, Norton TT (1991) Lateral geniculate nucleus: a review of its physiology and function. In: Leventhal A (ed) Vision and visual dysfunction vol. 4: the neural basis of visual function. Macmillan, London, pp 41–84
Casagrande VA, Harting JK, Hall WC, Diamond IT, Martin GF (1972) Superior colliculus of the tree shrew: a structural and functional subdivision into superficial and deep layers. Science 177:444–447
Cronin JE, Sarich VM (1980) Tupaiid and Archonta phylogeny: the macromolecular evidence. In: Luckett WP (ed) Comparative biology and evolutionary relationships of tree shrews. Plenum, New York, pp 293–312
Cusick CG, MacAvoy MG, Kaas JH (1985) Interhemispheric connections of cortical sensory areas in tree shrews. J Comp Neurol 235:111–128
Eayrs JT, Goodhead B (1959) Postnatal development of the cerebral cortex in the rat. J Anat 93:385–402
Elston GN (2001) Interlaminar differences in the pyramidal cell phenotype in cortical areas 7m and STP (the superior temporal polysensory area) of the macaque monkey. Exp Brain Res 138:141–152
Elston GN (2002) Cortical heterogeneity: implications for visual processing and polysensory integration. J Neurocytol 31:317–335
Elston GN (2003) Pyramidal cell heterogeneity in the visual cortex of the nocturnal New World owl monkey ( Aotus trivirgatus). Neuroscience 117:213–219
Elston GN, Garey LJ (2004) New research findings on the anatomy of the cerebral cortex of special relevance to anthropological questions. University of Queensland Printers, Brisbane, Australia
Elston GN, Rosa MGP (1997) The occipitoparietal pathway of the macaque monkey: comparison of pyramidal cell morphology in layer III of functionally related cortical visual areas. Cereb Cortex 7:432–452
Elston GN, Rosa MGP (1998) Morphological variation of layer III pyramidal neurones in the occipitotemporal pathway of the macaque monkey visual cortex. Cereb Cortex 8:278–294
Elston GN, Rosa MGP (2000) Pyramidal cells, patches, and cortical columns: a comparative study of infragranular neurons in TEO, TE, and the superior temporal polysensory area of the macaque monkey. J Neurosci 20: RC117(1–5)
Elston GN, Tweedale R, Rosa MGP (1999a) Cortical integration in the visual system of the macaque monkey: large scale morphological differences of pyramidal neurones in the occipital, parietal and temporal lobes. Proc R Soc Lond Ser B 266:1367–1374
Elston GN, Tweedale R, Rosa MGP (1999b) Cellular heterogeneity in cerebral cortex. A study of the morphology of pyramidal neurones in visual areas of the marmoset monkey. J Comp Neurol 415: 33–51
Elston GN, Elston A, Casagrande VA, Kaas J (2005) Regional specialization in pyramidal cell structure in the visual cortex of the Galago. An intracellular injection study with comparative notes on New World and Old World monkeys. Brain Behav Evol (in press)
Ferster D, Miller KD (2000) Neural mechanisms of orientation selectivity in the visual cortex. Annu Rev Neurosci 23:441–471
Graham J, Casagrande VA (1980) A light microscopic and electron microscopic study of the superficial layers of the superior colliculus of the tree shrew ( Tupaia glis). J Comp Neurol 191:133–151
Hubel DH, Wiesel TN (1968) Receptive fields and functional architecture of monkey striate cortex. J Physiol 195:215–243
Humphrey AL, Norton TT, Albano JE (1980a) Topographic organization of the orientation column system in the striate cortex of the tree shrew ( Tupaia glis). I. Microelectrode recording. J Comp Neurol 192:531–547
Humphrey AL, Skeen LC, Norton TT (1980b) Topographic organization of the orientation column system in the striate cortex of the tree shrew ( Tupaia glis). II. Deoxyglucose mapping. J Comp Neurol 192:549–566
Jacobs B, Scheibel AB (2002) Regional dendritic variation in primate cortical pyramidal cells. In: Schüz A, Miller R (eds) Cortical areas: unity and diversity. Taylor and Francis, London, pp 111–131
Jain N, Preuss TM, Kaas JH (1994) Subdivisions of the visual system labeled with the CAT-301 antibody in tree shrews. Vis Neurosci 11:731–741
Kaas JH, Hall WC, Killackey H, Diamond IT (1972) Visual cortex of the tree shrew ( Tupaia glis): architectonic subdivisions and representation of the visual field. Brain Res 42:491–496
Kisvárday ZF, Bonhoeffer T, Kim D-S, Eysel U (1996) Functional topography of horizontal neural networks in cat visual cortex (area 18). In: Aertesen A, Braitenberg B (eds) Brain theory—biological and computational theories. Elsevier, Amsterdam, pp 97–122
Kretz R, Rager G, Norton TT (1986) Laminar organization of ON and OFF regions and ocular dominance in the striate cortex of the tree shrew ( Tupaia belangeri). J Comp Neurol 251:135–145.
Lane RH, Allman J, Kaas JH (1971) Representation of the visual field in the superior colliculus of the grey squirrel ( Sciurus carolinensis) and the tree shrew ( Tupaia glis). Brain Res 26:277–292.
Le Gros Clark WE (1959) The Antecedents of Man. Edinburgh University Press, Edinburgh
Livingstone MS (1998) Mechanisms of direction selectivity in macaque V1. Neuron 20:509–526
Luckett WP (1980) The suggested evolutionary relationships and classification of tree shrews. In: Luckett WP (ed) Comparative biology and evolutionary relationships of tree shrews. Plenum, New York, pp 3–31
Lund JS, Fitzpatrick D, Humphrey AL (1985) The striate visual cortex of the tree shrew. In: Peters A, Jones EG (eds) Cerebral cortex. Plenum, New York, pp 157–205
Lund JS, Yoshioka T, Levitt JB (1993) Comparison of intrinsic connectivity in different areas of macaque monkey cerebral cortex. Cereb Cortex 3:148–162
Lyon DC, Jain N, Kaas JH (1998) Cortical connections of striate and extrastriate visual areas in tree shrews. J Comp Neurol 401:109–128
Lyon DC, Jain N, Kaas JH (2003) The visual pulvinar in tree shrews II. Projections of four nuclei to areas of visual cortex. J Comp Neurol 464:607–627
MacPhee RDE (1993) Summary. In: MacPhee RDE (ed) Primates and their relatives in phylogenetic perspective. Plenum, New York, pp 363–373
Mahe J (1976) Crâniométrie des lémuriéns analyses multivariables-phylogénie. Mem Mus Natl Hist Nat Ser C 32:1–342
Martin KAC, Whitteridge D (1984) The relationship of receptive field properties to the dendritic shape of neurons in cat striate cortex. J Physiol (Lond) 356:291–302
Martin RD (1990) Primate origins and evolution. Chapman and Hall, London
Mooser F, Bosking WH, Fitzpatrick D (2004) A morphological bias for orientation tuning in primary visual cortex. Nature Neurosci. 7:872–879.
Norton TT, Casagrande VA, Sherman SM (1977) Loss of Y-cells in the lateral geniculate nucleus of monocularly deprived tree shrews. Science 197:784–786
Norton TT, Rager G, Kretz R (1985) ON and OFF regions in layer IV of striate cortex. Brain Res 327:319–323
Nowak RM (1999) Walker’s primates of the world. Johns Hopkins University Press, Baltimore
Poirazi P, Mel B (2001) Impact of active dendrites and structural plasticity on the storage capacity of neural tissue. Neuron 29:779–796
Ringach DL, Shapley R, Hawken MJ (2002) Orientation selectivity in macaque V1: diversity and laminar dependence. J Neurosci 22:5639–5651
Rockland KS, Lund JS (1982) Widespread periodic intrinsic connections in the tree shrew visual cortex. Science 215:1532–1534
Rockland KS, Lund JS, Humphrey AL (1982) Anatomical banding of intrinsic connections in striate cortex of tree shrews ( Tupaia glis). J Comp Neurol 209:41–58
Sesma MA, Casagrande VA, Kaas JH (1984) Cortical connections in area 17 in tree shrews. J Comp Neurol 230:337–351
Sherman SM, Norton TT, Casagrande VA (1975) X- and Y-cells in the dorsal lateral geniculate nucleus of the tree shrew ( Tupaia glis). Brain Res 93:152–157
Sholl DA (1953) Dendritic organization in the neurons of the visual and motor cortices of the cat. J Anat 87:387–406
Simmons NB (1993) The importance of methods: Archontan phylogeny and cladistic analysis of morphological data. In: Ross PE (ed) Primates and their relatives in phylogenetic perspective. Plenum, New York, pp 1–61
Simpson GG (1945) The principles of classification and a classification of mammals. Bull Amer Mus Nat Hist 85:1–350
Springer MS, de Jong WW (2001) Phylogenetics. Which mammalian supertree to bark up? Science 291:1709–1711
Stepniewska I (2003) Comparative studies of pyramidal neurons in visual cortex of monkeys. In: Kaas JH, Collins C (eds) The primate visual system. CRC Press, Boca Raton, pp 53–80
Wong-Riley M (1979) Changes in the visual system of monocularly sutured or enucleated cats demonstrable with cytochrome oxidase histochemistry. Brain Res 171:11–28
Wong-Riley M, Norton TT (1988) Histochemical localization of cytochrome oxidase activity in the visual system of the tree shrew: normal patterns and the effect of retinal impulse blockade. J Comp Neurol 272:562–578
Acknowledgements
Thanks go to Iwona Stepniewska, Laura Trice, Laura Ferris, Julia Mavity-Hudson and Brendan Zietsch for technical help. Supported by grants from the JS McDonnell Foundation and the Australian National Health and Medical Research Council.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Elston, G.N., Elston, A., Casagrande, V. et al. Areal specialization of pyramidal cell structure in the visual cortex of the tree shrew: a new twist revealed in the evolution of cortical circuitry. Exp Brain Res 163, 13–20 (2005). https://doi.org/10.1007/s00221-004-2131-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-004-2131-7