Abstract
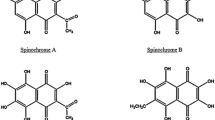
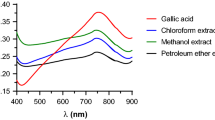
In the present study, the effects of operational parameters on adsorption and desorption capacity of polyhydroxylated 1,4-naphthoquinone (PHNQ) pigments on macroporous resin in static mode were evaluated. On this basis, a suitable operating condition was selected which recovered 83.96 ± 0.71 and 80.58 ± 1.48 % of PHNQ pigments from Glyptocidaris crenularis and Strongylocentrotus intermedius spines, respectively. Based on accurate molecular mass and ultraviolet–visible (UV–Vis) spectral data acquired by ultra-performance liquid chromatography hybrid quadrupole orthogonal acceleration time-of-flight mass spectrometer (UPLC Q-TOFMS), spinochrome E, spinochrome D and spinochrome B were tentatively identified in the pigment extract prepared from G. crenularis. In contrast, only spinochrome B was identified in the pigment extract from S. intermedius. Between the two extracts, the one from G. crenularis showed stronger DPPH radical scavenging ability and ferrous ion chelating capacity. In our opinion, the different PHNQ compounds existing in the two extracts resulted in the difference in antioxidant activity.





Similar content being viewed by others
References
Global Production Statistics (1950–2010) FAO. http://www.fao.org/fishery/statistics/global-production/query/en
Amarowicz R, Synowiecki J, Shahidi F (2012) Chemical composition of shells from red (Strongylocentrotus franciscanus) and green (Strongylocentrotus droebachiensis) sea urchin. Food Chem 133:822–826
Tyler A (1939) Crystalline echinochrome and spinochrome: their failure to stimulate the respiration of eggs and of sperm of Strongylocentrotus. Proc Natl Acad Sci USA 25:523–528
Goodwin TW, Srisukh S (1950) A study of the pigments of the sea-urchins, Echinus esculentus L. and Paracentrotus lividus Lamarck. Biochem J 47:69–76
Yoshida M (1959) Naphthoquinone pigments in Psammechinus miliaris. J Mar Biol Assoc UK 38:455–460
Moore RE, Singh H, Scheuer PJ (1966) Isolation of eleven new Spin chromes from Echinoids of the genus Echinothrix. J Org Chem 31:3645–3660
Singh I, Ogata RT, Moore RE, Chang CWJ, Scheuer PJ (1968) Electronic spectra of substituted naphthoquinones. Tetrahedron 24:6053–6073
Amarowicz R, Synowiecki J, Shahidi F (1994) Sephadex LH-20 separation of pigments from shells of red sea urchin (Strongylocentrotus franciscanus). Food Chem 51:227–229
Anderson HA, Mathieson JW, Thomson RH (1969) Distribution of spinochrome pigments in echinoids. Comp Biochem Physiol 28:333–345
Mischenko NP, Fedoreyev SA, Pokhilo ND, Anufriev VP, Denisenko VA, Glazunov VP (2005) Echinamines A and B, first aminated hydroxynaphthazarins from the sea urchin Scaphechinus mirabilis. J Nat Prod 68:1390–1393
Yakubovskaya AY, Pokhilo ND, Mishchenko NP, Anufriev VF (2007) Spinazarin and ethylspinazarin, pigments of the sea urchin Scaphechinus mirabilis. Russ Chem Bul 56:819–822
Moore RE, Singh H, Chang CWJ, Scheuer PJ (1966) Sodium borohydride reduction of spinochrome A. removal of phenolic hydroxyls in the naphthazarin system. J Org Chem 31:3638–3645
Anufriev VP, Novikov VL, Maximov OB, Elyakov GB, Levitsky DO, Lebedev AV, Sadretdinov SM, Shvilkin AV, Afonskaya NI, Ruda MY, Cherpachenko NM (1998) Synthesis of some hydroxynaphthazarins and their cardioprotective effects under ischemia-reperfusion in vivo. Bioorg Med Chem Lett 8:587–592
Pokhilo ND, Shuvalova MI, Lebedko MV, Sopelnyak GI, Yakubovskaya AY, Mischenko NP, Fedoreyev SA, Anufriev VP (2006) Synthesis of echinamines A and B, the first aminated hydroxynaphthazarins produced by the sea urchin Scaphechinus mirabilis and its analogues. J Nat Prod 69:1125–1129
Service M, Wardlaw AC (1984) Echinochrome-A as a bactericidal substance in the coelomic fluid of Echinus esculentus (L.). Comp Biochem Physiol B: Biochem Mol Biol 79:161–165
Lebedev AV, Levitskaya EL, Tikhonova EV, Ivanova MV (2001) Antioxidant properties, autooxidation, and mutagenic activity of echinochrome a compared with its etherified derivative. Biochem (Mosc) 66:885–893
Mishchenko NP, Fedoreev SA, Bagirova VL (2003) Histochrome: a new original domestic drug. Pharm Chem J 37:48–52
Lebedev AV, Ivanova MV, Levitsky DO (2005) Echinochrome, a naturally occurring iron chelator and free radical scavenger in artificial and natural membrane systems. Life Sci 76:863–875
Kuwahara R, Hatate H, Yuki T, Murata H, Tanaka R, Hama Y (2009) Antioxidant property of polyhydroxylated naphthoquinone pigments from shells of purple sea urchin Anthocidaris crassispina. LWT-Food Sci Technol 42:1296–1300
Kuwahara R, Hatate H, Chikami A, Murata H, Kijidani Y (2010) Quantitative separation of antioxidant pigments in purple sea urchin shells using a reversed-phase high performance liquid chromatography. LWT-Food Sci Technol 43:1185–1190
Zhou DY, Qin L, Zhu BW, Wang XD, Tan H, Yang JF, Li DM, Dong XP, Wu HT, Sun LM, Li XL, Murata Y (2011) Extraction and antioxidant property of polyhydroxylated naphthoquinone pigments from spines of purple sea urchin Strongylocentrotus nudus. Food Chem 129:1591–1597
Zhou DY, Zhu BW, Wang XD, Qin L, Li DM, Miao L, Murata Y (2012) Stability of polyhydroxylated 1,4-naphthoquinone pigment recovered from spines of sea urchin Strongylocentrotus nudus. Int J Food Sci Technol 47:1479–1486
McClendon JF (1912) Echinochrome, a red substance in sea urchins. J Biol Chem 11:435
Utkina NK, Shchedrin AP, Maksimov OB (1976) A new binaphthoquinone from strongylocentrotus intermedius. Chem Nat Compd 12:387–389
Kol′tsova EA, Denisenko VA, Maksimov OB (1978) Quinoid pigments of echinodermata V. Pigments of the sea urchin Strongy locentrotus dröebachiensis. Chem Nat Compd 14:371–374
Kol′tsova EA, Krasovskaya NP (2009) Quinoid pigments from the sea urchin Toxopneustes pileolus. Chem Nat Compd 45:427–428
Zhou XF, Wen KW, Yang XW, Huang RM, Dong G, Yang B, Sun JF, Liu YH (2010) Chemical constituents from the sea urchin Glyptocidaris crenularis. Biochem Syst Ecol 38:103–105
Wang YN, Feng NS, Li Q, Ding J, Zhan YY, Chang YQ (2012) Isolation and characterization of bacteria associated with a syndrome disease of sea urchin Strongylocentrotus intermedius in North China. Aquac Res. (in press) (doi:10.1111/j.1365-2109.2011.03073.x)
Chen Y, Wang MF, Rosen RT, Ho CT (1999) 2,2-diphenyl-1-picrylhydrazyl radical-scavenging active components from Polygonum multiflorum thunb. J Agric Food Chem 47:2226–2228
Hsu B, Coupar IM, Ng K (2006) Antioxidant activity of hot water extract from the fruit of the Doum palm, Hyphaene thebaica. Food Chem 98:317–328
Lebedev AV, Ivanova MV, Ruuge EK (2003) How do calcium ions induce free radical oxidation of hydroxy-1,4-naphthoquinone? Ca2+ stabilizes the naphthosemiquinone anion-radical of echinochrome A. Arch Biochem Biophys 413:191–198
Cao GH, Sofic E, Prior RL (1997) Antioxidant and prooxidant behavior of flavonoids: structure-activity relationships. Free Radic Bio Med 22:749–760
Herrero-Martínez JM, Sanmartin M, Rosés M, Bosch E, Ràfols C (2005) Determination of dissociation constants of flavonoids by capillary electrophoresis. Electrophoresis 26:1886–1895
Tsujimoto M, Horie M, Honda H, Takara K, Nishiguchi K (2009) The structure–activity correlation on the inhibitory effects of flavonoids on cytochrome P450 3A activity. Biol Pharm Bull 32:671–676
Acknowledgments
This work was financially supported by “The National High Technology Research and Development Program of China (863 Program) (No. 2011AA100803)” and “The Research Start-up Project for Doctor Funded by Liaoning Science and Technology Department (No. 20091002)”.
Conflict of interest
None.
Compliance with Ethics Requirements
This article does not contain any studies with human or animal subjects.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, DM., Zhou, DY., Zhu, BW. et al. Extraction, structural characterization and antioxidant activity of polyhydroxylated 1,4-naphthoquinone pigments from spines of sea urchin Glyptocidaris crenularis and Strongylocentrotus intermedius . Eur Food Res Technol 237, 331–339 (2013). https://doi.org/10.1007/s00217-013-1996-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-013-1996-8