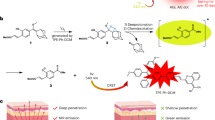
Abstract
In this study, we developed a colorimetric ATP assay based on the ATP-induced aggregation of Au nanoparticles (AuNPs). This aggregation modified the local surface plasmon resonance (LSPR) of the AuNPs, which was used to detect and localize ATP in cells via dark-field imaging. The AuNP aggregation process involved the reaction of two types of functionalized AuNPs with each other: tetrazine-modified AuNPs (Au3-N4) and asymmetrically functionalized trans-cyclooctene-modified AuNPs (Au1-(E)-cyclooctene). This cycloaddition reaction occurs without the need for a catalyst such as the Cu ions that are used in the “click” reactions often employed in assays of this type. Initially, we asymmetrically functionalized both types of AuNPs and let them dimerize, which permitted us to explore the resulting wavelength shift in the LSPR of the AuNPs. Then, to facilitate the specific recognition of ATP, a designed DNA (DNA1) containing an ATP aptamer sequence was attached to carboxyl polystyrene microbeads (MBs). After attaching a different DNA (DNA2, which hybridizes with DNA1) to Au1-(E)-cyclooctene, the assay probe MB/DNA1/DNA2/Au1-(E)-cyclooctene (MB/Au1) was generated. While bound to MB/DNA1, the DNA2/Au1-(E)-cyclooctene cannot react with Au3-N4 due to steric hindrance from the MB. However, in the presence of ATP, the probe MB/Au1 dissociates, and the resulting free DNA2/Au1-(E)-cyclooctene can then react with the Au3-N4, leading to the formation of AuNP aggregates. Dark-field microscopy (DFM) images showed that the LSPR of the AuNPs shifted from the green region (AuNP monomers) to the orange-red region (AuNP aggregates) in the presence of intracellular ATP. Moreover, the AuNP aggregates were found to exhibit significant photothermal effects under 808-nm laser irradiation. Upon introducing the probe MB/Au1 and Au3-N4 into HeLa cells in vitro and in vivo, and then irradiating the cells with a 808-nm NIR laser, the resulting AuNP aggregates showed promising photothermal cancer therapy performance. This assay therefore has the potential to be widely used for the identification and determination of nanoparticles in biological DFM and in tumor theranostics.

Graphical abstract





Similar content being viewed by others
References
Anker JN, Hall WP, Lyandres O, Shah NC, Zhao J, Van Duyne RP. Biosensing with plasmonic nanosensors. Nat Mater. 2008;7:442–53.
Nanand F, Yan Z. Sorting metal nanoparticles with dynamic and tunable optical driven forces. Nano Lett. 2018;18:4500–5.
Jain PK, Huang X, El-Sayed IH, El-Sayed MA. Noble metals on the nanoscale: optical and photothermal properties and some applications in imaging, sensing, biology, and medicine. Acc Chem Res. 2008;41:1578–86.
Xiong B, Zhou R, Hao J, Jia Y, He Y, Yeung ES. Highly sensitive sulphide mapping in live cells by kinetic spectral analysis of single Au-Ag core-shell nanoparticles. Nat Commun. 2013;4:1708–17.
Li K, Qin W, Li F, Zhao X, Jiang B, Wang K, et al. Nanoplasmonic imaging of latent fingerprints and identification of cocaine. Angew Chem Int Ed. 2013;52:11542–5.
Sheikholeslami S, Jun Y, Jain PK, Alivisatos AP. Coupling of optical resonances in a compositionally asymmetric plasmonic nanoparticle dimer. Nano Lett. 2010;10:2655–60.
Roller E, Argyropoulos C, Högele A, Liedl T, Pilo-Pais M. Plasmon-exciton coupling using DNA templates. Nano Lett. 2016;16:5962–6.
Chen T, Hongand Y, Reinhard BM. Probing DNA stiffness through optical fluctuation analysis of plasmon rulers. Nano Lett. 2015;15:5349–57.
Sönnichsen C, Reinhard BM, Liphardt J, Alivisatos AP. A molecular ruler based on plasmon coupling of single gold and silver nanoparticles. Nat Biotechnol. 2005;23:741–5.
Qi F, Han Y, Ye Z, Liu H, Wei L, Xiao L. Color-coded single-particle pyrophosphate assay with dark-field optical microscopy. Anal Chem. 2018;90:11146–53.
Li T, Xu X, Zhang G, Lin R, Chen Y, Li C, et al. Nonamplification sandwich assay platform for sensitive nucleic acid detection based on AuNPs enumeration with the dark-field microscope. Anal Chem. 2016;88:4188–91.
Wang X, Cui Y, Irudayaraj J. Single-cell quantification of cytosine modifications by hyperspectral dark-field imaging. ACS Nano. 2015;9:11924–32.
Wonner K, Evers MV, Tschulik K. The electrochemical dissolution of single silver nanoparticles enlightened by hyperspectral dark-field microscopy. Electrochim Acta. 2019;301:458–64.
John R. Energy transfer from adenosine triphosphate. J Phys Chem B. 2006;110:6987–90.
Nakano S, Fukuda M, Tamura T, Sakaguchi R, Nakata E, Morii T. Simultaneous detection of ATP and GTP by covalently linked fluorescent ribonucleopeptide sensors. J Am Chem Soc. 2013;135:3465–73.
Zhao Q, Zhang Z, Tang Y. A new conjugated polymer-based combination probe for ATP detection using a multisite-binding and FRET strategy. Chem Commun. 2017;53:9414–7.
Cheng D, Li Y, Wang J, Sun Y, Jin L, Li CX, et al. Fluorescence and colorimetric detection of ATP based on a strategy of self-promoting aggregation of a water-soluble polythiophene derivative. Chem Commun. 2015;51:8544–6.
You J, Lu C, Kumar ASK, Tseng W. Cerium(III)-directed assembly of glutathione-capped gold nanoclusters for sensing and imaging of alkaline phosphatase-mediated hydrolysis of adenosine triphosphate. Nanoscale. 2018;10:17691–8.
Xie H, Chai Y, Yuan Y, Yuan R. Highly effective molecule converting strategy based on enzyme-free dual recycling amplification for ultrasensitive electrochemical detection of ATP. Chem Commun. 2017;53:8368–71.
Hickey DP, Lim K, Cai R, Patterson AR, Yuan M, Sahin S, et al. Pyrene hydrogel for promoting direct bioelectrochemistry: ATP-independent electroenzymatic reduction of N2. Chem Sci. 2018;9:5172–7.
Liu JH, Li RS, Yuan B, Wang J, Li YF, Huang CZ. Mitochondria-targeting single-layered graphene quantum dots with dual recognition sites for ATP imaging in living cells. Nanoscale. 2018;10:17402–8.
Wang L, Yuan L, Zeng X, Peng J, Ni Y, Er JC, et al. A multisite-binding switchable fluorescent probe for monitoring mitochondrial ATP level fluctuation in live cells. Angew Chem Int Ed. 2016;55:1773–6.
Yuan L, Wang X, Fang Y, Liu C, Jiang D, Wo X, et al. Digitizing gold nanoparticle-based colorimetric assay by imaging and counting single nanoparticles. Anal Chem. 2016;88:2321–6.
Vieira EG, Miguel RB, Silva DR, Fazzi RB, Couto RA, Marin JH, et al. Functionalized nanoparticles as adjuvant to increase the cytotoxicity of metallodrugs toward tumor cells. New J Chem. 2019;43:386–98.
Chen Z, Tan L, Hu L, Zhang Y, Wang S, Lv F. Real colorimetric thrombin aptasensor by masking surfaces of catalytically active gold nanoparticles. ACS Appl Mater Interfaces. 2016;8:102–8.
Wang J, Wu L, Ren J, Qu X. Visual detection of telomerase activity with a tunable dynamic range by using a gold nanoparticle probe-based hybridization protection strategy. Nanoscale. 2014;6:1661–6.
Zeng C, Lu N, Wen Y, Liu G, Zhang R, Zhang J, et al. Engineering nanozymes using DNA for catalytic regulation. ACS Appl Mater Interfaces. 2019;11:1790–9.
Wu T, Chang C, Vaillant J, Bruyant A, Lin C. DNA biosensor combining single-wavelength colorimetry and a digital lock-in amplifier with in a smartphone. Lab Chip. 2016;16:4527–33.
Liu X, Wu Z, Zhang Q, Zhao W, Zong C, Gai H. Single gold nanoparticle-based colorimetric detection of picomolar mercury ion with dark-field microscopy. Anal Chem. 2016;88:2119–24.
Parnsubsakul A, Oaew S, Surareungchai W. Zwitterionic peptide-capped gold nanoparticles for colorimetric detection of Ni2+. Nanoscale. 2018;10:5466–73.
Farida A, Anna D, Gamze K, Melike S, Mustafa C, Rawil F. Simultaneous intracellular detection of plasmonic and non-plasmonic nanoparticles using dark-field hyperspectral microscopy. Bull Chem Soc Jpn. 2018;91:1640–5.
Sönnichsen C, Reinhard BM, Liphardt J, Alivisatos AP. A molecular ruler based on plasmon coupling of single gold and slver nanoparticles. Nat Biotechnol. 2005;23:741–5.
Yang K, Luo H, Zeng M, Jiang Y, Li J, Fu X. Intracellular pH-triggered, targeted drug delivery to cancer cells by multifunctional envelope-type mesoporous silica nanocontainers. ACS Appl Mater Interfaces. 2015;7:17399–407.
Guo Y, Li S, Wang Y, Zhang S. Diagnosis-therapy integrative systems based on magnetic RNA nanoflowers for co-drug delivery and targeted therapy. Anal Chem. 2017;89:2267–74.
Funding
This work was supported by the National Natural Science Foundation of China (21575056), the Natural Science Foundation of Shandong Province of China, (ZR2016JL010), and the Primary Research and Development Plan of Shandong Province (2018GSF118172).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that there is no conflict of interest.
Ethics approval and consent to participate
All animal experimental procedures and techniques were approved by the Animal Ethics Committee of East China Normal University, and methods were carried out in accordance with the approved guidelines and laws.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 1029 kb)
Rights and permissions
About this article
Cite this article
Liu, F., Guo, Y., Hu, Y. et al. Intracellular dark-field imaging of ATP and photothermal therapy using a colorimetric assay based on gold nanoparticle aggregation via tetrazine/trans-cyclooctene cycloaddition. Anal Bioanal Chem 411, 5845–5854 (2019). https://doi.org/10.1007/s00216-019-01966-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-019-01966-0