Abstract
Retention indices for 124 polycyclic aromatic hydrocarbons (PAHs) and 62 methyl-substituted (Me-) PAHs were determined using normal-phase liquid chromatography (NPLC) on a aminopropyl (NH2) stationary phase. PAH retention behavior on the NH2 phase is correlated to the total number of aromatic carbons in the PAH structure. Within an isomer group, non-planar isomers generally elute earlier than planar isomers. MePAHs generally elute slightly later but in the same region as the parent PAHs. Correlations between PAH retention behavior on the NH2 phase and PAH thickness (T) values were investigated to determine the influence of non-planarity for isomeric PAHs with four to seven aromatic rings. Correlation coefficients ranged from r = 0.19 (five-ring peri-condensed molecular mass (MM) 252 Da) to r = −0.99 (five-ring cata-condensed MM 278 Da). In the case of the smaller PAHs (MM ≤ 252 Da), most of the PAHs had a planar structure and provided a low correlation. In the case of larger PAHs (MM ≥ 278 Da), nonplanarity had a significant influence on the retention behavior and good correlation between retention and T was obtained for the MM 278 Da, MM 302 Da, MM 328 Da, and MM 378 Da isomer sets.

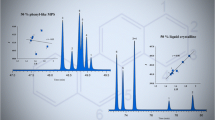
NPLC separation of the three-, four-, five-, and six-ring PAH isomers with different number of aromatic carbon atoms and degrees of non-planarity (Thickness, T). The inserted figure plots the number of aromatic carbon atoms vs. the log I value for the 124 parent PAHs.







Similar content being viewed by others
References
Canha N, Lopes I, Vicente ED, Vicente AM, Bandowe BAM, Almeida SM, et al. Mutagenicity assessment of aerosols in emissions from domestic combustion processes. Environ Sci Pollut Res. 2016;23(11):10799–807. doi:10.1007/s11356-016-6292-2.
Olson GM, Meyer BM, Portier RJ. Assessment of the toxic potential of polycyclic aromatic hydrocarbons (PAHs) affecting gulf menhaden (Brevoortia patronus) harvested from waters impacted by the bp deepwater horizon spill. Chemosphere. 2016;145:322–8. doi:10.1016/j.chemosphere.2015.11.087.
de Souza MR, da Silva FR, de Souza CT, Niekraszewicz L, Dias JF, Premoli S, et al. Evaluation of the genotoxic potential of soil contaminated with mineral coal tailings on snail Helix aspersa. Chemosphere. 2015;139:512–7. doi:10.1016/j.chemosphere.2015.07.071.
Wise SA, Sander LC, Schantz MM. Analytical methods for determination of polycyclic aromatic hydrocarbons (PAHs) - a historical perspective on the 16 US EPA priority pollutant PAHs. Polycycl Aromat Compd. 2015;35(2–4):187–247. doi:10.1080/10406638.2014.970291.
Poster DL, Schantz MM, Sander LC, Wise SA. Analysis of polycyclic aromatic hydrocarbons (PAHs) in environmental samples: a critical review of gas chromatographic (GC) methods. Anal Bioanal Chem. 2006;386(4):859–81. doi:10.1007/s00216-006-0771-0.
Wise SA, Benner BA, Liu HC, Byrd GD, Colmsjö A. Separation and identification of polycyclic aromatic hydrocarbon isomers of molecular-weight 302 in complex-mixtures. Anal Chem. 1988;60(7):630–7. doi:10.1021/ac00158a006.
Wise SA, Benner BA, Byrd GD, Chesler SN, Rebbert RE, Schantz MM. Determination of polycyclic aromatic-hydrocarbons in a coal-tar standard reference material. Anal Chem. 1988;60(9):887–94. doi:10.1021/ac00160a012.
Wise SA, Schantz MM, Benner BA, Hays MJ, Schiller SB. Certification of polycyclic aromatic-hydrocarbons in a marine sediment standard reference material. Anal Chem. 1995;67(7):1171–8. doi:10.1021/ac00103a006.
Regueiro J, Matamoros V, Thibaut R, Porte C, Bayona JM. Use of effect-directed analysis for the identification of organic toxicants in surface flow constructed wetland sediments. Chemosphere. 2013;91(8):1165–75. doi:10.1016/j.chemosphere.2013.01.023.
Wise SA, Chesler SN, Hertz HS, Hilpert LR, May WE. Chemically-bonded aminosilane stationary phase for high-performance liquid-chromatographic separation of polynuclear aromatic-compounds. Anal Chem. 1977;49(14):2306–10. doi:10.1021/ac50022a049.
May WE, Wise SA. Liquid-chromatographic determination of polycyclic aromatic-hydrocarbons in air particulate extracts. Anal Chem. 1984;56(2):225–32. doi:10.1021/ac00266a024.
Wise SA, Benner BA, Chesler SN, Hilpert LR, Vogt CR, May WE. Characterization of the polycyclic aromatic-hydrocarbons from 2 standard reference material air particulate samples. Anal Chem. 1986;58(14):3067–77. doi:10.1021/ac00127a036.
Wise SA, Deissler A, Sander LC. Liquid chromatographic determination of polycyclic aromatic hydrocarbon isomers of molecular weight 278 and 302 in environmental standard reference materials. Polycycl Aromat Compd. 1993;3(3):169–84. doi:10.1080/10406639308047869.
Hertz HS, Brown JM, Chesler SN, Guenther FR, Hilpert LR, May WE, et al. Determination of individual organic-compounds in shale oil. Anal Chem. 1980;52(11):1650–7. doi:10.1021/ac50061a027.
Olsson P, Sadiktsis I, Holmback J, Westerholm R. Class separation of lipids and polycyclic aromatic hydrocarbons in normal phase high performance liquid chromatography - a prospect for analysis of aromatics in edible vegetable oils and biodiesel exhaust particulates. J Chromatogr A. 2014;1360:39–46. doi:10.1016/j.chroma.2014.07.064.
Fang ML, Getzinger GJ, Cooper EM, Clark BW, Garner LVT, Di Giulio RT, et al. Effect-directed analysis of elizabeth river porewater: developmental toxicity in zebrafish (Danio rerio). Environ Toxicol Chem. 2014;33(12):2767–74. doi:10.1002/etc.2738.
Marvin CH, McCarry BE, Lundrigan JA, Roberts K, Bryant DW. Bioassay-directed fractionation of pah of molecular mass 302 in coal tar-contaminated sediment. Sci Total Environ. 1999;231(2–3):135–44. doi:10.1016/s0048-9697(99)00096-0.
Swistek M, Weber JV. A separative tool for analyzing heavy coal and petroleum residues - the extrography. Analusis. 1991;19(7):191–7.
Gilgenast E, Boczkaj G, Przyjazny A, Kaminski M. Sample preparation procedure for the determination of polycyclic aromatic hydrocarbons in petroleum vacuum residue and bitumen. Anal Bioanal Chem. 2011;401(3):1059–69. doi:10.1007/s00216-011-5134-9.
Popl M, Dolansky V, Mostecky J. Influence of molecular-structure of aromatic-hydrocarbons on their adsorptivity on silica-gel. J Chromatogr. 1976;117(1):117–27. doi:10.1016/s0021-9673(00)81072-9.
Popl M, Dolansky V, Mostecky J. Influence of molecular-structure of aromatic-hydrocarbons on their adsorptivity on alumina. J Chromatogr. 1974;91:649–58. doi:10.1016/s0021-9673(01)97945-2.
Wise SA, Bonnett WJ, May WE. Normal- and reversed-phase liquid chromatographic separations of polycyclic aromatic hydrocarbons. In: ABaAJ D, editor. Polynuclear aromatic hydrocarbons: chemistry and biological effects. Columbus, OH: Battelle Press; 1980. p. 791–806.
Wilson WB, Hayes HV, Sander LC, Campiglia AD, Wise SA. Retention behavior of polycyclic aromatic sulfur heterocycles and their alkyl-substituted derivatives via normal-phase liquid chromatography. Anal Bioanal chem. 2017; in preparation.
Wilson WB, Hayes HV, Sander LC, Campiglia AD, Wise SA. Qualitative characterization of srm 1597a for polycyclic aromatic hydrocarbons and some methyl-substituted derivatives via normal-phase liquid chromatography and gas chromatography/mass spectrometry. Anal Bioanal Chem. 2017; in preparation.
L. C. Sander, S. A. Wise, Polycyclic Aromatic Hydrocarbon Structure Index, NIST Special Publication 922 (https://www.nist.gov/mml/csd/special-publication-922-polycyclic-aromatic-hydrocarbon-structure-index), Washington, DC, 1997 (accessed 17.01.03).
Wise SA, Jinno SLCK. Chromatographic separatiosn based on molecular recognition. Wiley-VCH. 1997:1–64.
Wilson WB, Sander LC, de Alda ML, Lee ML, Wise SA. Retention behavior of isomeric polycyclic aromatic sulfur heterocycles in reversed-phase liquid chromatography. J Chromatogr A. 2016;1461:107–19. doi:10.1016/j.chroma.2016.07.064.
Wilson WB, Sander LC, de Alda ML, Lee ML, Wise SA. Retention behavior of alkyl-substituted polycyclic aromatic sulfur heterocycles in reversed-phase liquid chromatography. J Chromatogr A. 2016;1461:120–30. doi:10.1016/j.chroma.2016.07.065.
Sun M, Feng JJ, Luo CN, Liu X, Jiang SX. Quinolinium ionic liquid-modified silica as a novel stationary phase for high-performance liquid chromatography. Anal Bioanal Chem. 2014;406(11):2651–8. doi:10.1007/s00216-014-7680-4.
Grizzle PL, Thomson JS. Liquid chromatographic separation of aromatic hydrocarbons with chemically bonded (2,4-dinitroanilinopropyl)silica. Anal Chem. 1982;54(7):1071–8. doi:10.1021/ac00244a013.
Thomson JS, Reynolds JW. Separation of aromatic hydrocarbons using bonded-phase charge-transfer liquid chromatography. Anal Chem. 1984;56(13):2434–41. doi:10.1021/ac00277a040.
Lubcke-von Varel U, Streck G, Brack W. Automated fractionation procedure for polycyclic aromatic compounds in sediment extracts on three coupled normal-phase high-performance liquid chromatography columns. J Chromatogr A. 2008;1185(1):31–42. doi:10.1016/j.chroma.2008.01.055.
Lubke M, Lequere JL, Barron D. Normal-phase high-performance liquid-chromatography of volatile compounds - selectivity and mobile-phase effects on polar bonded silica. J Chromatogr A. 1995;690(1):41–54. doi:10.1016/0021-9673(94)01048-j.
Gautam UG, Shundo A, Gautam MP, Takafuji M, Ihara H. High retentivity and selectivity for polycyclic aromatic hydrocarbons with poly(4-vinylpyridine)-grafted silica in normal-phase high-performance liquid chromatography. J Chromatogr A. 2008;1189(1–2):77–82. doi:10.1016/j.chroma.2007.12.018.
Jiang P, Lucy CA. Retentivity, selectivity and thermodynamic behavior of polycyclic aromatic hydrocarbons on charge-transfer and hypercrosslinked stationary phases under conditions of normal phase high performance liquid chromatography. J Chromatogr A. 2016;1437:176–82. doi:10.1016/j.chroma.2016.02.014.
Miller MJ, Miller JC. Statistics and chemometrics for analytical cehmistry. 6th ed. New York: Prentice-Hall, Inc.; 2000.
Sander LC, Wise SA. Synthesis and characterization of polymeric C18 stationary phases for liquid chromatography. Anal Chem. 1984;56(3):504–10. doi:10.1021/ac00267a047.
Sander LC, Wise SA. Shape selectivity in reversed-phase liquid chromatography for the separation of planar and non-planar solutes. J Chromatogr A. 1993;656(1–2):335–51. doi:10.1016/0021-9673(93)80808-L.
Sander LC, Wise SA. Determination of column selectivity toward polycyclic aromatic hydrocarbons. J High Resolut Chromatogr. 1988;11(5):383–7. doi:10.1002/jhrc.1240110505.
Acknowledgements
H. V. Hayes and A. D. Campiglia acknowledge financial support from The Gulf of Mexico Research Initiative (Grant 231617-00). The views expressed are those of the authors and do not necessarily reflect the view of this organization.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Disclaimer
Certain commercial equipment or materials are identified in this paper to specify adequately the experimental procedure. Such identification does not imply recommendation or endorsement by the National Institute of Standards and Technology, nor does it imply that the materials or equipment identified are necessarily the best available for the purpose.
Electronic supplementary material
ESM 1
(PDF 2.05 mb)
Rights and permissions
About this article
Cite this article
Wilson, W.B., Hayes, H.V., Sander, L.C. et al. Normal-phase liquid chromatography retention behavior of polycyclic aromatic hydrocarbon and their methyl-substituted derivatives on an aminopropyl stationary phase. Anal Bioanal Chem 409, 5291–5305 (2017). https://doi.org/10.1007/s00216-017-0474-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-017-0474-8