Abstract
Vancomycin is an important glycopeptide antibiotic which is used to treat serious infections caused by Gram-positive bacteria. However, during the last years, a tremendous rise in vancomycin resistances, especially among Enterococci, was reported, making fast diagnostic methods inevitable. In this contribution, we apply Raman spectroscopy to systematically characterize vancomycin-enterococci interactions over a time span of 90 min using a sensitive Enterococcus faecalis strain and two different vancomycin concentrations above the minimal inhibitory concentration (MIC). Successful action of the drug on the pathogen could be observed already after 30 min of interaction time. Characteristic spectral changes are visualized with the help of multivariate statistical analysis (linear discriminant analysis and partial least squares regressions). Those changes were employed to train a statistical model to predict vancomycin treatment based on the Raman spectra. The robustness of the model was tested using data recorded by an independent operator. Classification accuracies of >90 % were obtained for vancomycin concentrations in the lower range of a typical trough serum concentration recommended for most patients during appropriate vancomycin therapy. Characterization of drug–pathogen interactions by means of label-free spectroscopic methods, such as Raman spectroscopy, can provide the knowledge base for innovative and fast susceptibility tests which could speed up microbiological analysis as well as finding applications in novel antibiotic screenings assays.

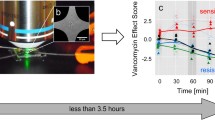
E. faecalis is incubated with vancomycin and characterized by means of Raman spectroscopy after different time points. Characteristic spectral changes reveal efficient vancomycin-enterococci-interaction




Similar content being viewed by others
References
Levine DP (2006) Vancomycin: a history. Clin Infect Dis 42(Supplement 1):S5–S12. doi:10.1086/491709
Sood S, Malhotra M, Das BK, Kapil A (2008) Enterococcal infections & antimicrobial resistance. Indian J Med Res 128(2):111–121
Van Bambeke F, Van Laethem Y, Courvalin P, Tulkens PM (2004) Glycopeptide antibiotics from conventional molecules to new derivatives. Drugs 64(9):913–936. doi:10.2165/00003495-200464090-00001
Kahne D, Leimkuhler C, Wei L, Walsh C (2005) Glycopeptide and lipoglycopeptide antibiotics. Chem Rev 105(2):425–448. doi:10.1021/cr030103a
Arias CA, Murray BE (2012) The rise of the Enterococcus: beyond vancomycin resistance. Nat Rev Microbiol 10(4):266–278. doi:10.1038/nrmicro2761
Werner G, Strommenger B, Witte W (2008) Acquired vancomycin resistance in clinically relevant pathogens. Futur Microbiol 3(5):547–562. doi:10.2217/17460913.3.5.547
Kristich CJ, Rice LB, Arias CA (2014) Enterococcal infection—treatment and antibiotic resistance. Enterococci: from commensals to leading causes of drug resistant infection. Massachusetts Eye and Ear Infirmary, Boston
Kudo M, Nomura T, Yomoda S, Tanimoto K, Tomita H (2014) Nosocomial infection caused by vancomycin-susceptible multidrug-resistant Enterococcus faecalis over a long period in a university hospital in Japan. Microbiol Immunol 58(11):607–614. doi:10.1111/1348-0421.12190
Gastmeier P, Schröder C, Behnke M, Meyer E, Geffers C (2014) Dramatic increase in vancomycin-resistant enterococci in Germany. J Antimicrob Chemother 69(6):1660–1664. doi:10.1093/jac/dku035
Fernandes SC, Dhanashree B (2013) Drug resistance & virulence determinants in clinical isolates of Enterococcus species. Indian J Med Res 137(5):981–985
Noble WC, Virani Z, Cree RG (1992) Co-transfer of vancomycin and other resistance genes from Enterococcus faecalis NCTC 12201 to Staphylococcus aureus. FEMS Microbiol Lett 72(2):195–198
Hegstad K, Mikalsen T, Coque TM, Werner G, Sundsfjord A (2010) Mobile genetic elements and their contribution to the emergence of antimicrobial resistant Enterococcus faecalis and Enterococcus faecium. Clin Microbiol Infect 16(6):541–554. doi:10.1111/j.1469-0691.2010.03226.x
de Niederhäusern S, Bondi M, Messi P, Iseppi R, Sabia C, Manicardi G, Anacarso I (2011) Vancomycin-resistance transferability from VanA enterococci to Staphylococcus aureus. Curr Microbiol 62(5):1363–1367. doi:10.1007/s00284-011-9868-6
Brehm-Stecher BF, Johnson EA (2004) Single-cell microbiology: tools, technologies, and applications. Microbiol Mol Biol Rev 68(3):538–559. doi:10.1128/mmbr.68.3.538-559.2004
Jorgensen JH, Ferraro MJ (2009) Antimicrobial susceptibility testing: a review of general principles and contemporary practices. Clin Infect Dis 49(11):1749–1755. doi:10.1086/647952
Palladino S, Kay ID, Flexman JP, Boehm I, Costa AM, Lambert EJ, Christiansen KJ (2003) Rapid detection of vanA and vanB genes directly from clinical specimens and enrichment broths by real-time multiplex PCR assay. J Clin Microbiol 41(6):2483–2486
Waldeisen JR, Wang T, Mitra D, Lee LP (2011) A real-time PCR antibiogram for drug-resistant sepsis. PLoS One 6(12), e28528. doi:10.1371/journal.pone.0028528
Gousia P, Economou V, Bozidis P, Papadopoulou C (2015) Vancomycin-resistance phenotypes, vancomycin-resistance genes, and resistance to antibiotics of enterococci isolated from food of animal origin. Foodborne Pathog Dis. doi:10.1089/fpd.2014.1832
Machen A, Drake T, Wang YF (2014) Same day identification and full panel antimicrobial susceptibility testing of bacteria from positive blood culture bottles made possible by a combined lysis-filtration method with MALDI-TOF VITEK mass spectrometry and the VITEK2 system. PLoS One 9(2), e87870. doi:10.1371/journal.pone.0087870
Jung JS, Popp C, Sparbier K, Lange C, Kostrzewa M, Schubert S (2014) Evaluation of matrix-assisted laser desorption ionization-time of flight mass spectrometry for rapid detection of beta-lactam resistance in Enterobacteriaceae derived from blood cultures. J Clin Microbiol 52(3):924–930. doi:10.1128/jcm.02691-13
Lange C, Schubert S, Jung J, Kostrzewa M, Sparbier K (2014) Quantitative matrix-assisted laser desorption ionization-time of flight mass spectrometry for rapid resistance detection. J Clin Microbiol 52(12):4155–4162. doi:10.1128/jcm.01872-14
Gauthier C, St-Pierre Y, Villemur R (2002) Rapid antimicrobial susceptibility testing of urinary tract isolates and samples by flow cytometry. J Med Microbiol 51(3):192–200
Jarzembowski T, Jozwik A, Wisniewska K, Witkowski J (2010) Flow cytometry approach study of Enterococcus faecalis vancomycin resistance by detection of Vancomycin@FL binding to the bacterial cells. Curr Microbiol 61(5):407–410. doi:10.1007/s00284-010-9628-z
Seo JY, Kim PW, Lee JH, Song JH, Peck KR, Chung DR, Kang CI, Ki CS, Lee NY (2011) Evaluation of PCR-based screening for vancomycin-resistant enterococci compared with a chromogenic agar-based culture method. J Med Microbiol 60(Pt 7):945–949. doi:10.1099/jmm.0.029777-0
Chan WS, Chan TM, Lai TW, Chan JF, Lai RW, Lai CK, Tang BS (2015) Complementary use of MALDI-TOF MS and real-time PCR-melt curve analysis for rapid identification of methicillin-resistant staphylococci and VRE. J Antimicrob Chemother 70(2):441–447. doi:10.1093/jac/dku411
Kloss S, Kampe B, Sachse S, Rösch P, Straube E, Pfister W, Kiehntopf M, Popp J (2013) Culture independent Raman spectroscopic identification of urinary tract infection pathogens: a proof of principle study. Anal Chem 85(20):9610–9616. doi:10.1021/ac401806f
Münchberg U, Rösch P, Bauer M, Popp J (2014) Raman spectroscopic identification of single bacterial cells under antibiotic influence. Anal Bioanal Chem 406(13):3041–3050. doi:10.1007/s00216-014-7747-2
Harz M, Kiehntopf M, Stöckel S, Rösch P, Straube E, Deufel T, Popp J (2009) Direct analysis of clinical relevant single bacterial cells from cerebrospinal fluid during bacterial meningitis by means of micro-Raman spectroscopy. J Biophotonics 2(1-2):70–80. doi:10.1002/jbio.200810068
Mathey R, Dupoy M, Espagnon I, Leroux D, Mallard F, Novelli-Rousseau A (2015) Viability of 3h grown bacterial micro-colonies after direct Raman identification. J Microbiol Methods 109:67–73. doi:10.1016/j.mimet.2014.12.002
Schröder U-C, Ramoji A, Glaser U, Sachse S, Leiterer C, Csaki A, Hübner U, Fritzsche W, Pfister W, Bauer M, Popp J, Neugebauer U (2013) Combined dielectrophoresis-Raman setup for the classification of pathogens recovered from the urinary tract. Anal Chem 85(22):10717–10724. doi:10.1021/ac4021616
Maquelin K, Kirschner C, Choo-Smith LP, van den Braak N, Endtz HP, Naumann D, Puppels GJ (2002) Identification of medically relevant microorganisms by vibrational spectroscopy. J Microbiol Methods 51(3):255–271. doi:10.1016/s0167-7012(02)00127-6
Kirschner C, Maquelin K, Pina P, Ngo Thi NA, Choo-Smith LP, Sockalingum GD, Sandt C, Ami D, Orsini F, Doglia SM, Allouch P, Mainfait M, Puppels GJ, Naumann D (2001) Classification and identification of enterococci: a comparative phenotypic, genotypic, and vibrational spectroscopic study. J Clin Microbiol 39(5):1763–1770. doi:10.1128/JCM.39.5.1763-1770.2001
Rösch P, Harz M, Schmitt M, Peschke KD, Ronneberger O, Burkhardt H, Motzkus HW, Lankers M, Hofer S, Thiele H, Popp J (2005) Chemotaxonomic identification of single bacteria by micro-Raman spectroscopy: application to clean-room-relevant biological contaminations. Appl Environ Microbiol 71(3):1626–1637. doi:10.1128/AEM.71.3.1626-1637.2005
Harz M, Rösch P, Popp J (2009) Vibrational spectroscopy--a powerful tool for the rapid identification of microbial cells at the single-cell level. Cytometry A 75(2):104–113. doi:10.1002/cyto.a.20682
Kastanos EK, Kyriakides A, Hadjigeorgiou K, Pitris C (2010) A novel method for urinary tract infection diagnosis and antibiogram using Raman spectroscopy. J Raman Spectrosc 41(9):958–963. doi:10.1002/jrs.2540
Lu XN, Al-Qadiri HM, Lin MS, Rasco BA (2011) Application of mid-infrared and Raman spectroscopy to the study of bacteria. Food Bioprocess Tech 4(6):919–935. doi:10.1007/s11947-011-0516-8
Kloss S, Rösch P, Pfister W, Kiehntopf M, Popp J (2015) Toward culture-free Raman spectroscopic identification of pathogens in ascitic fluid. Anal Chem 87(2):937–943. doi:10.1021/ac503373r
Schröder U-C, Beleites C, Assmann C, Glaser U, Hübner U, Pfister W, Fritzsche W, Popp J, Neugebauer U (2015) Detection of vancomycin resistances in enterococci within 3 (1/2) hours. Sci Rep 5:8217. doi:10.1038/srep08217
Madiyar FR, Bhana S, Swisher LZ, Culbertson CT, Huang X, Li J (2015) Integration of a nanostructured dielectrophoretic device and a surface-enhanced Raman probe for highly sensitive rapid bacteria detection. Nanoscale 7(8):3726–3736. doi:10.1039/c4nr07183b
Neugebauer U, Schmid U, Baumann K, Holzgrabe U, Ziebuhr W, Kozitskaya S, Kiefer W, Schmitt M, Popp J (2006) Characterization of bacterial growth and the influence of antibiotics by means of UV resonance Raman spectroscopy. Biopolymers 82(4):306–311. doi:10.1002/bip.20447
Neugebauer U, Schmid U, Baumann K, Ziebuhr W, Kozitskaya S, Holzgrabe U, Schmitt M, Popp J (2007) The influence of fluoroquinolone drugs on the bacterial growth of S. epidermidis utilizing the unique potential of vibrational spectroscopy. J Phys Chem A 111(15):2898–2906. doi:10.1021/jp0678397
Jung GB, Nam SW, Choi S, Lee GJ, Park HK (2014) Evaluation of antibiotic effects on Pseudomonas aeruginosa biofilm using Raman spectroscopy and multivariate analysis. Biomed Opt Express 5(9):3238–3251. doi:10.1364/boe.5.003238
Moritz TJ, Polage CR, Taylor DS, Krol DM, Lane SM, Chan JW (2010) Evaluation of Escherichia coli cell response to antibiotic treatment by use of Raman spectroscopy with laser tweezers. J Clin Microbiol 48(11):4287–4290. doi:10.1128/JCM.01565-10
Heidari Torkabadi H, Bethel CR, Papp-Wallace KM, de Boer PA, Bonomo RA, Carey PR (2014) Following drug uptake and reactions inside Escherichia coli cells by Raman microspectroscopy. Biochemistry 53(25):4113–4121. doi:10.1021/bi500529c
Athamneh AI, Alajlouni RA, Wallace RS, Seleem MN, Senger RS (2014) Phenotypic profiling of antibiotic response signatures in Escherichia coli using Raman spectroscopy. Antimicrob Agents Chemother 58(3):1302–1314. doi:10.1128/aac.02098-13
Liu TY, Tsai KT, Wang HH, Chen Y, Chen YH, Chao YC, Chang HH, Lin CH, Wang JK, Wang YL (2011) Functionalized arrays of Raman-enhancing nanoparticles for capture and culture-free analysis of bacteria in human blood. Nat Commun 2:538. doi:10.1038/ncomms1546
Stöckel S, Walter A, Boßecker A, Meisel S, Ciobotă V, Schumacher W, Rösch P, Popp J (2011) Identification and characterization of microorganisms by vibrational spectroscopy. In: Popp J, Tuchin VV, Chiou A, Heinemann SH (eds) Handbook of biophotonics, vol 2, Photonics for Health Care. John Wiley & Sons, Weinheim, pp 105–142
McCreery RL (2000) Raman spectroscopy for chemical analysis, vol 157, Chemical analysis. John Wiley & Sons, New York
R Core Team (2014) R: a language and environment for statistical computing. R version 3.0.3 (2014-03-06). R Foundation for Statistical Computing, Vienna
Beleites C, Sergo V (2014) hyperSpec: a package to handle hyperspectral data sets in R. R package version 0.98-20140220
Wickham H (2009) ggplot2: elegant graphics for data analysis. Springer, New York. doi:10.1007/978-0-387-98141-3
Venables WN, Ripley BD (2002) Modern applied statistics with S. Statistics and computing, 4th edn. Springer, New York
Lasch P, Hermelink A, Naumann D (2009) Correction of axial chromatic aberrations in confocal Raman microspectroscopic measurements of a single microbial spore. Analyst 134(6):1162–1170. doi:10.1039/b822553b
Barker M, Rayens W (2003) Partial least squares for discrimination. J Chemometrics 17(3):166–173
Næs T, Mevik B-H (2001) Understanding the collinearity problem in regression and discriminant analysis. J Chemometrics 15(4):413–426
Beleites C (2014) cbmodels: Collection of "combined" models: PCA-LDA, PLS-LDA, etc. R package version 0.5-20140225
Mevik B-H, Wehrens R, Liland KH (2013) pls: Partial Least Squares and Principal Component regression. R package version 2.4-3
Beleites C, Neugebauer U, Bocklitz T, Krafft C, Popp J (2013) Sample size planning for classification models. Anal Chim Acta 760 (0):25-33. doi:http://dx.doi.org/10.1016/j.aca.2012.11.007
Edelstein EM, Rosenzweig MS, Daneo-Moore L, Higgins ML (1980) Unit cell hypothesis for Streptococcus faecalis. J Bacteriol 143(1):499–505
Koch AL, Higgins ML (1984) Control of wall band splitting in Streptococcus faecalis. J Gen Microbiol 130(4):735–745
Shlaes DM, Bouvet A, Devine C, Shlaes JH, al-Obeid S, Williamson R (1989) Inducible, transferable resistance to vancomycin in Enterococcus faecalis A256. Antimicrob Agents Chemother 33(2):198–203
Swenson JM, Clark NC, Sahm DF, Ferraro MJ, Doern G, Hindler J, Jorgensen JH, Pfaller MA, Reller LB, Weinstein MP et al (1995) Molecular characterization and multilaboratory evaluation of Enterococcus faecalis ATCC 51299 for quality control of screening tests for vancomycin and high-level aminoglycoside resistance in enterococci. J Clin Microbiol 33(11):3019–3021
The European Committee on Antimicrobial Susceptibility Testing (2015) Breakpoint tables for interpretation of MICs and zone diameters. http://www.eucast.org
Rybak MJ, Lomaestro BM, Rotschafer JC, Moellering RC, Craig WA, Billeter M, Dalovisio JR, Levine DP (2009) Vancomycin therapeutic guidelines: a summary of consensus recommendations from the infectious diseases society of america, the american society of health-system pharmacists, and the society of infectious diseases pharmacists. Clin Infect Dis 49(3):325–327. doi:10.1086/600877
Große C, Bergner N, Dellith J, Heller R, Bauer M, Mellmann A, Popp J, Neugebauer U (2015) Label-free imaging and spectroscopic analysis of intracellular bacterial infections. Anal Chem 87(4):2137–2142. doi:10.1021/ac503316s
Naumann D, Labischinski H, Rönspeck W, Barnickel G, Bradaczek H (1987) Vibrational spectroscopic analysis of LD-sequential, bacterial cell wall peptides: an IR and Raman study. Biopolymers 26(6):795–817. doi:10.1002/bip.360260603
Notingher I, Verrier S, Romanska H, Bishop AE, Polak JM, Hench LL (2002) In situ characterisation of living cells by Raman spectroscopy. Spectroscopy 16(2):43–51. doi:10.1155/2002/408381
Beleites C, Baumgartner R, Bowman C, Somorjai R, Steiner G, Salzer R, Sowa MG (2005) Variance reduction in estimating classification error using sparse datasets. Chemometrics Intellig Lab Syst 79(1–2):91–100. doi:10.1016/j.chemolab.2005.04.008
Kohavi R (1995) A study of cross-validation and bootstrap for accuracy estimation and model selection. In: Proceedings of the 14th international joint conference on Artificial intelligence, Montreal, Quebec, Canada. Morgan Kaufmann Publishers Inc., San Francisco, CA, USA, pp 1137-1143
Acknowledgments
Financial support of the BMBF via the Integrated Research and Treatment Center “Center for Sepsis Control and Care” (FKZ 01EO1002) and via the Carl Zeiss Stiftung is highly acknowledged. We thank A. Saupe for the VITEK® measurements as well as Martin Gnauck and Steffen Wolf for recording the scanning electron microscope (SEM) image (graphical abstract).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in the topical collection Raman4Clinics with guest editors Jürgen Popp and Christoph Krafft.
Cora Assmann and Johanna Kirchhoff contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 116 kb)
Rights and permissions
About this article
Cite this article
Assmann, C., Kirchhoff, J., Beleites, C. et al. Identification of vancomycin interaction with Enterococcus faecalis within 30 min of interaction time using Raman spectroscopy. Anal Bioanal Chem 407, 8343–8352 (2015). https://doi.org/10.1007/s00216-015-8912-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-015-8912-y