Abstract
The interacting quantum atoms (IQA) energy partition has given important insights about different systems and processes in theoretical chemistry. Given its intrinsic dependence on first- and second-order density matrices, IQA is only cleanly defined within wavefunction methods. This means that, despite the importance of density functional theory (DFT) in electronic structure methods, a neat IQA–DFT implementation is not straightforward. This work addresses this issue through a new implementation of IQA within the Kohn–Sham formalism of DFT in conjunction with hybrid and non-hybrid functionals that contributes further to that already presented (Maxwell et al. in Phys Chem Chem Phys, 2016. doi:10.1039/C5CP07021J). For this purpose, we use additive exchange-correlation (xc) energies, defined within the IQA approach, to scale the one- and two-atom terms of the Kohn–Sham xc energy. This leads to an exact partition of the xc DFT energy of a molecule into intra-atomic and inter-atomic contributions. The suggested method is illustrated with several molecules together with some of the most popular local and hybrid DFT functionals. Overall, we anticipate that the approach put forward in this work will prove useful in getting further insights of phenomena in chemistry which are properly described with DFT .
Similar content being viewed by others
Notes
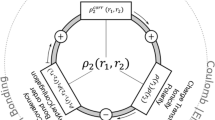
The McWeeny normalization criterion, \(\int _{\infty } \int _{\infty } \rho _2(\varvec{r}_1,\varvec{r}_2) {\mathrm{d}}\varvec{r}_1 {\mathrm{d}}\varvec{r}_2=N(N-1)\), has been used in Eq. 3.
References
Stone AJ (2013) The theory of intermolecular forces. Oxford University Press, Oxford
Hayes IC, Stone AJ (1984) Mol Phys 53:83
Hayes IC, Stone AJ (1984) Mol Phys 53:69
London F (1937) Trans Faraday Soc 33:8
Longuet-Higgins HC (1965) Discuss Faraday Soc 40:7
Jeziorski B, Moszynski R, Szalewicz K (1994) Chem Rev 94:1887
Misquitta AJ, Podeszwa R, Jeziorski B, Szalewicz K (2005) J Chem Phys 123:214103
Hobza P, Müller-Dethlefs K (2010) Non-covalent interactions: theory and experiment. Cambridge University Press, Cambridge
Hesselmann A, Jansen G, Schütz M (2006) J Chem Phys 122:014103
Morokuma K (1971) J Chem Phys 55:1236
Kitaura K, Morokuma K (1976) Int J Quantum Chem 10:325
Bickelhaupt FM, Evert Jan Baerends EJ (2007) Rev Comput Chem 15:1
Ziegler T, Rauk A (1977) Theor Chem Acc 46:1
Su P, Li H (2009) J Chem Phys 131:014102
Glendening ED, Streitwieser J (1994) J Chem Phys 100:2900
Weinhold F (1997) J Mol Struct 399:191
Reed AR, Weinhold F (1983) J Chem Phys 78:4066
Reed AR, Weinhold F, Curtiss LA, Pochatko DJ (1986) J Chem Phys 84:5687
Reed AR, Curtiss LA, Weinhold F (1988) Chem Rev 88:899
Blanco MA, Martín Pendás A, Francisco E (2005) J Chem Theory Comput 1:1096
Francisco E, Martín Pendás A, Blanco MA (2006) J Chem Theory Comput 2:90
Tognetti V, Joubert L (2014) Phys Chem Chem Phys 16:14539
Maxwell P, Martín Pendás A, Popelier PLA (2016) Phys Chem Chem Phys. doi:10.1039/C5CP07021J
Parr RG, Yang W (1989) Density-functional theory of atoms and molecules. Oxford University Press, New York
Becke A (1993) J Chem Phys 98:5648
Stephens PJ, Devlin FJ, Chabalowski CF, Frisch MJ (1994) J Phys Chem 98:11623
McWeeny R (1992) Methods of molecular quantum mechanics, 2nd edn. Academic Press, London
Löwdin PO (1955) Phys Rev 97:1474
Martín Pendás A, Francisco E (2015) Promolden. A qtaim/iqa code (unpublished). A QTAIM/IQA code (Unpublished)
Schmidt MW, Baldridge KK, Boatz JA, Elbert ST, Gordon MS, Jensen JH, Koseki S, Matsunaga N, Nguyen KA, Su SJ, Windus TL, Dupuis M, Montgomery JA (1993) J Comput Chem 14:1347
Dirac PAM (1930) Proc Camb Philos Soc 26:376
Vosko SH, Wilk L, Nusair M (1980) Can J Phys 58:1980
Perdew JP, Burke K, Ernzerhof M (1996) Phys Rev Lett 77:3865
Perdew JP, Burke K, Ernzerhof M (1997) Phys Rev Lett 78:1396(E)
Lebedev VI, Laikov DN (1999) Dokl Math 59:477
Francisco E, Martín Pendás A (2015) Imolint (Unpublished)
Bader RFW (1990) Atoms in molecules. Oxford University Press, Oxford
Martín Pendás A, Blanco MA, Francisco E (2004) J Chem Phys 120:4581
Marques MAL, Oliveira MJT, Burnus T (2012) Comput Phys Commun 183:2272
Rodríguez JI, Ayers PA, Götz W, Castillo-Alvarado FL (2009) J Chem Phys 131:021101
Acknowledgments
The authors thank the Spanish MINECO, Grant CTQ2012-31174, for financial support. T. R-R gratefully acknowledges computer time from DGTIC/UNAM (project SC16-1-IG-99) and funding from CONACyT-México and DGAPA/UNAM, Grants 253776 and IN209715 respectively. Additionally, T. R.-R. is also thankful to David Vázquez Cuevas, Gladys Cortés Romero and Magdalena Aguilar Araiza for technical support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published as part of the special collection of articles “Festschrift in honour of A. Vela”.
Rights and permissions
About this article
Cite this article
Francisco, E., Casals-Sainz, J.L., Rocha-Rinza, T. et al. Partitioning the DFT exchange-correlation energy in line with the interacting quantum atoms approach. Theor Chem Acc 135, 170 (2016). https://doi.org/10.1007/s00214-016-1921-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-016-1921-x