Abstract
Rationale
Previous studies have demonstrated behaviors indicative of anxiolysis in rats pretreated with the nociceptin receptor (opioid receptor like-1, ORL-1) agonist, Ro64-6198.
Objectives
The aim of this study was to examine the effects of Ro64-6198 in anxiety models across three species: rat, guinea pig, and mouse. In addition, the receptor specificity of Ro64-6198 was studied, using the ORL-1 receptor antagonist, J-113397, and ORL-1 receptor knockout (KO) mice. Finally, neurological studies examined potential side effects of Ro64-6198 in the rat and mouse.
Results
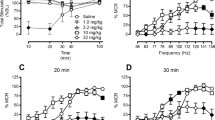
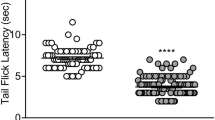
Ro64-6198 (3–10 mg/kg) increased punished responding in a rat conditioned lick suppression test similarly to chlordiazepoxide (6 mg/kg). This effect of Ro64-6198 was attenuated by J-113397 (10 mg/kg), but not the mu opioid antagonist, naltrexone (3 mg/kg). In addition, Ro64-6198 (1–3 mg/kg) reduced isolation-induced vocalizations in rat and guinea pig pups. Ro64-6198 (3 mg/kg) increased the proportion of punished responding in a mouse Geller–Seifter test in wild-type (WT) but not ORL-1 KO mice, whereas diazepam (1–5.6 mg/kg) was effective in both genotypes. In rats, Ro64-6198 reduced locomotor activity (LMA) and body temperature and impaired rotarod, beam walking, and fixed-ratio (FR) performance at doses of 10–30 mg/kg, i.e., three to ten times higher than an anxiolytic dose. In WT mice, Ro64-6198 (3–10 mg/kg) reduced LMA and rotarod performance, body temperature, and FR responding, but these same measures were unaffected in ORL-1 KO mice. Haloperidol (0.3–3 mg/kg) reduced these measures to a similar extent in both genotypes. These studies confirm the potent, ORL-1 receptor-mediated, anxiolytic-like effects of Ro64-6198, extending the findings across three species. Ro64-6198 has target-based side effects, although the magnitude of these effects varies across species.






Similar content being viewed by others
References
Calo G, Guerrini R, Rizzi A, Salvadori S, Regoli D (2000) Pharmacology of nociceptin and its receptor: a novel therapeutic target. Br J Pharmacol 129:1261–1283
Champion HC, Kadowitz PJ (1997) Nociceptin, an endogenous ligand for the ORL1 receptor, has novel hypotensive activity in the rat. Life Sci 60:PL241–PL245
Devine DP, Reinscheid RK, Monsma FJ Jr, Civelli O, Akil H (1996a) The novel neuropeptide orphanin FQ fails to produce conditioned place preference or aversion. Brain Res 727:225–229
Devine DP, Taylor L, Reinscheid RK, Monsma FJ Jr, Civelli O, Akil H (1996b) Rats rapidly develop tolerance to the locomotor inhibiting effects of the novel neuropeptide orphanin FQ. Neurochem Res 21:1387–1396
Dorow R (1987) FG 7142 and its anxiety-inducing effects in humans. Br J Clin Pharmacol 23:781–782
Fernandez F, Misilmeri MA, Felger JC, Devine DP (2004) Nociceptin/orphanin FQ increases anxiety-related behavior and circulating levels of corticosterone during neophobic tests of anxiety. Neuropsychopharmacology 29:59–71
Gavioli EC, Rae GA, Calo G, Guerrini R, De Lima TC (2002) Central injections of nocistatin or its C-terminal hexapeptide exert anxiogenic-like effect on behaviour of mice in the plus-maze test. Br J Pharmacol 136:764–772
Gerlai R (1996) Gene-targetting studies of mammalian behaviour: is it the mutation or the background genotype? Trends Neurosci 19:177–181
Griebel G, Perrault G, Sanger DJ (1999) Orphanin FQ a novel neuropeptide with anti-stress-like activity. Brain Res 836:221–224
Hashiba E, Lambert DG, Jenck F, Wichmann J, Smith G (2002) Characterisation of the non-peptide nociceptin receptor agonist, Ro 64-6198 in Chinese hamster ovary cells expressing recombinant human nociceptin receptors. Life Sci 70:1719–1725
Higgins GA, Grottick AJ, Ballard TM, Richards JG, Messer J, Takeshima H, Pauly-Evers M, Jenck F, Adam G, Wichmann J (2001) Influence of the selective ORL1 receptor agonist, Ro 64-6198, on rodent neurological function. Neuropharmacology 41:97–107
Higgins GA, Kew JNC, Richards JG, Takeshima H, Jenck F, Adam G, Wichmann J, Kemp JA, Grottick AJ (2002) A combined pharmacological and genetic approach to investigate the role of orphanin FQ in learning and memory. Eur J Neurosci 15:911–922
Jenck F, Moreau J-L, Martin JR, Kilpatrick GJ, Reinscheid RK, Monsma FJ, Nothacker H-P, Civelli O (1997) Orphanin FQ acts as an anxiolytic to attenuate behavioural responses to stress. Proc Natl Acad Sci U S A 94:14854–14858
Jenck F, Wichmann J, Dautzenberg FM, Moreau J-L, Ouagazzal AM, Martin JR, Lundstrom K, Cesura AM, Poli SM, Roever S, Kolczewski S, Adam G, Kilpatrick GJ (2000) A synthetic agonist at the orphanin FQ/nociceptin receptor ORL1: anxiolytic profile in the rat. Proc Natl Acad Sci U S A 97:4938–4943
Kapusta DR, Chang JK, Kenigs VA (1999) Central administration of [Phe1psi(CH2-NH)Gly2]nociceptin(1-13)-NH2 and orphanin FQ/nociceptin (OFQ/N) produce similar cardiovascular and renal responses in conscious rats. J Pharmacol Exp Ther 289:173–180
Kawamoto H, Ozaki S, Itoh Y, Miyaji M, Arai S, Nakashima H, Kato T, Ohta H, Iwasawa Y (1999) Discovery of the first potent and selective small molecule opioid receptor-like (ORL1) antagonist: 1-[(3R,4R)-1-cyclooctylmethyl-3-hydroxymethyl-4-piperidyl]-3-ethyl-1, 3-dihydro-2H-benzimidazol-2-one (J-113397). J Med Chem 42:5061–5063
Kazdoba TM, Del Vecchio RA, Hyde LA (2003) Automated evaluation of sensitivity to foot shock in mice: inbred strain differences and analgesic effect of morphine. Soc Neurosci Abstr 29:836.18
Kehne JH, Coverdale S, McCloskey TC, Hoffman DC, Cassella JV (2000) Effects of the CRF(1) receptor antagonist, CP 154,526, in the separation-induced vocalization anxiolytic test in rat pups. Neuropharmacology 39:1357–1367
Kyuhou S, Gemba H (1999) Injection of orphanin FQ/nociceptin into the periaqueductal gray suppresses the forebrain-elicited vocalization in the guinea pig. Neurosci Lett 260:113–116
Le Pen G, Wichmann J, Moreau J-L, Jenck F (2002) The orphanin receptor agonist Ro 64-6198 does not induce place conditioning in rats. Neuroreport 13:451–454
Marti M, Mela F, Veronesi C, Guerrini R, Salvadori S, Federici M, Mercuri NB, Rizzi A, Franchi G, Beani L, Bianchi C, Morari M (2004) Blockade of nociceptin/orphanin FQ receptor signaling in rat substantia nigra pars reticulate stimulates nigrostriatal dopaminergic transmission and motor behaviour. J Neurosci 24:6659–6666
McElroy JF, Ward KA, Zeller KL, Jones KW, Gilligan PJ, He L, Lelas S (2002) The CRF(1) receptor antagonist DMP696 produces anxiolytic effects and inhibits the stress-induced hypothalamic–pituitary–adrenal axis activation without sedation or ataxia in rats. Psychopharmacology 165:86–92
Meunier JC, Mollereau C, Toll L, Suaudeau C, Moisand C, Alvinerie P, Butour JL, Guillemot JC, Ferrara P, Monsarrat B, Mazarguil H, Vassart G, Parmentier M, Costentin J (1995) Isolation and structure of the endogenous agonist of opioid receptor-like ORL1 receptor. Nature 377:532–535
Mogil JS, Pasternak GW (2001) The molecular and behavioural pharmacology of the orphanin FQ/nociceptin peptide and receptor family. Pharmacol Rev 53:381–415
Molewijk HE, Hartog K, van der Poel AM, Mos J, Olivier B (1996) Reduction of guinea pig pup isolation calls by anxiolytic and antidepressant drugs. Psychopharmacology 128:31–38
Nishi M, Houtani T, Noda Y, Mamiya T, Sato K, Doi T, Kuno J, Takeshima H, Nukuda T, Nabeshima T, Yamashita T, Noda T, Sugimoto T (1997) Unrestrained nociceptive response and disregulation of hearing ability in mice lacking the nociceptin/orphanin FQ receptor. EMBO J 16:1858–1864
Olivier B, Molewijk E, van Oorschot R, van der Heyden J, Ronken E, Mos J (1998) Rat pup ultrasonic vocalization: effects of benzodiazepine receptor ligands. Eur J Pharmacol 358:117–128
Ozaki S, Kawamoto H, Itoh Y, Miyaji M, Azuma T, Ichikawa D, Nambu H, Iguchi T, Iwasawa Y, Ohta H (2000) In vitro and in vivo pharmacological characterisation of J-113397, a potent and selective non-peptidyl ORL1 receptor antagonist. Eur J Pharmacol 402:45–53
Pomonis JD, Billington CJ, Levine AS (1996) Orphanin FQ, agonist of orphan receptor ORL1, stimulates feeding in rats. Neuroreport 8:369–371
Recker MD, Higgins GA (2004) The ORL-1 receptor agonist, Ro 64-6198, produces a discriminative stimulus in rats distinct from that of a mu, kappa and delta opioid receptor agonist cue. J Pharmacol Exp Ther 311:652–658
Redrobe JP, Calo G, Regoli D, Quirion R (2002) Nociceptin receptor antagonists display antidepressant-like properties in the mouse forced swimming test. Naunyn Schmiedebergs Arch Pharmacol 365:164–167
Reinscheid RK, Nothacker HP, Bourson A, Ardati A, Henningsen RA, Bunzow JR, Grandy DK, Langen H, Monsma FJ Jr, Civelli O (1995) Orphanin FQ: a neuropeptide that activates an opioid like G protein-coupled receptor. Science 270:792–794
Rizzi D, Bigoni R, Rizzi A, Jenck F, Wichmann J, Guerrini R, Regoli D, Calo G (2001) Effects of Ro 64-6198 in nociceptin/orphanin FQ-sensitive isolated tissues. Naunyn Schmiedebergs Arch Pharmacol 363:551–555
Rodgers RJ (1997) Animal models of ‘anxiety’: where next? Behav Pharmacol 8:477–496
Silva AJ, Simpson EM, Takahashi JS, Lipp H-P, Nakanishi S, Wehner JM, Geise KP, Tully T, Abel T, Chapman PF, Fox K, Grant S, Itahora S, Lathe R, Mayford M, McNamara JO, Morris RJ, Piciotto M, Roder J, Shin H-S, Slesinger PA, Storm DR, Stryker MP, Tonegawa S, Wang Y, Wolfer DP (1997) Mutant mice and neuroscience: recommendations concerning genetic background. Banbury conference on genetic background in mice. Neuron 19:755–759
Smith PA, Moran TD (2001) The nociceptin receptor as a potential target in drug design. Drug News Perspect 14:335–345
Snyder SH, Pasternak GW (2003) Historical review: opioid receptors. Trends Pharmacol Sci 24:198–205
Sulaiman MR, Niklasson M, Tham R, Dutia MB (1999) Modulation of vestibular function by nociceptin/orphanin FQ: an in vivo and in vitro study. Brain Res 828:74–82
Varty GB, Pond A, Hodgson R, Grzelak M, Guthrie D, Lu S, Morgan C, Higgins GA (2003) Determination of the anxiolytic vs. side-effect profile of a nociceptin NOP1 agonist and metabotrophic mGluR5 antagonists using rodent models of anxiety and neurological function. Soc Neurosci Abstr 29:959.11
Wichmann J, Adam G, Rover S, Hennig M, Scalone M, Cesura AM, Dautzenberg FM, Jenck F (2000) Synthesis of 1S,3aS.-8-2,3,3a,4,5,6-hexahydro-1H-phenalen-1-yl.-1-phenyl-1,3,8-triaza-spiro[4.5]decan-4-one, a potent and selective orphanin FQ receptor agonist with anxiolytic-like properties. Eur J Med Chem 35:839–851
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Varty, G.B., Hyde, L.A., Hodgson, R.A. et al. Characterization of the nociceptin receptor (ORL-1) agonist, Ro64-6198, in tests of anxiety across multiple species. Psychopharmacology 182, 132–143 (2005). https://doi.org/10.1007/s00213-005-0041-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-005-0041-4