Abstract
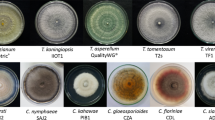
Bakanae disease is an emerging problem for the Basmati rice cultivation in India. Forty-seven endophytes isolated earlier along with three Talaromyces flavus isolates evaluated against Fusaium fujikuroi [Nirenberg] bakanae pathogen [isolate F250] through dual culture and enzymatic assays. Out of 50 isolates, 6 isolates namely, Tf1, Tf2, Tf3, Fusarium equiseti, Fusarium sp. and Trichoderma sp. produced good inhibitory results under in vitro conditions and were proceeded with in planta studies and conducted microscopic studies and real-time PCR assays. Microscopic studies revealed that the defense response system of plants was activated to a longer extent in bioagent treatments, since the number of live nuclei (DAPI staining) and green stained live plant cells (FDA staining) were higher as seen in treated plants when compared to pathogen-inoculated and uninoculated control when observed under confocal laser scanning microscopy. The analysis of cell cycle-related genes expressed during the ROS activity showed increased expression of the cell cycle-related genes involved. The selected isolates were also tested under glasshouse for disease inhibition studies. F. equiseti, Fusarium sp. and Trichoderma sp. gave a disease inhibition of, 87%, 66% and 94%, respectively. Tf2 and Tf1 isolate dominantly inhibited the disease with 95% whereas Tf3 also inhibited successfully with 70%. Through the results of our study, we can deduce that the T. flavus (Tf1, Tf2, Tf3) isolates and the endophytes F. equiseti, Fusarium sp. and Trichoderma sp. may represent an important biocontrol agent to control the bakanae disease of rice and also implicated that could further be befitting to capitalize them for field evaluations.





Similar content being viewed by others
References
Ajit NS, Verma R, Shanmugam V (2006) Extracellular chitinases of fluorescent pseudomonads antifungal to Fusarium oxysporum f. sp. dianthi causing carnation wilt. Curr Microbiol 52(4):310–316
Barnett S, Zhao S, Ballard R, Franco C (2017) Selection of microbes for control of Rhizoctonia root rot on wheat using a high throughput pathosystem. Biol Control 113:45–57. https://doi.org/10.1016/j.biocontrol.2017.07.003
Bashyal BM, Aggarwal R (2013) Molecular identification of Fusarium spp. associated with bakanae disease of rice in India. Indian J Agric Sci 83(1):72–77
Bashyal BM, Aggarwal R, Banerjee S, Gupta S, Sharma S (2014) Pathogenicity, ecology and genetic diversity of the Fusarium spp. associated with an emerging bakanae disease of rice (Oryza sativa L.) in India. In: Kharwar et al (eds) Microbial diversity and biotechnology in food security. Springer, New York, pp 307–314
Bashyal BM, Aggarwal R, Sharma S, Gupta S, Singh UB (2016) Single and combined effects of three Fusarium species associated with rice seeds on the severity of bakanae disease of rice. J Plant Pathol 98(2):405–412
Bateman IJ, Carson RT, Day B, Hanemann M, Hanley N, Hett T, Swanson J (2002) Economic valuation with stated preference techniques: a manual
Chhipa H, Kaushik N (2017) Fungal and bacterial diversity isolated from Aquilaria malaccensis tree and soil, induces agarospirol formation within 3 months after artificial infection. Front Microbiol 8:1286. https://doi.org/10.3389/fmicb.2017.01286
Crissman HA, Stevenson AP, Kissane RJ, Tobey RA (1979) Techniques for quantitative staining of cellular DNA for flow cytometric analysis. In: Melamed MR, Mullaney PF, Mendelsohn ML (eds) Flow cytometry and sorting. John Wiley and Sons Inc., New York, pp 243–261
Doke N (1983) Involvement of superoxide anion generation in the hypersensitive response of potato tuber tissues to infection with an incompatible race of Phytophthora infestans and to the hyphal wall components. Physiol Plant Pathol 23:345–357
Edidin M (1970) A rapid, quantitative fluorescence assay for cell damage by cytotoxic antibodies. J Immunol 104(5):1303–1306
Ezaki B, Gardner RC, Ezaki Y, Matsumoto H (2000) Expression of aluminum-induced genes in transgenic Arabidopsis plants can ameliorate aluminum stress and/or oxidative stress. Plant Physiol 122:657–665
Galbraith DW, Harkins KR, Maddox JM, Ayres NM, Sharma DP, Firoozabady E (1983) Rapid flow cytometric analysis of the cell cycle in intact plant tissues. Science 220:1049–1051
Goswami RS, Kistler HC (2004) Heading for disaster: Fusarium graminearum on cereal crops. Mol Plant Pathol 5:515–525. https://doi.org/10.1111/j.1364-3703.2004.00252.x
Grant JJ, Yun BW, Loake GJ (2000) Oxidative burst and cognate redox signalling reported by luciferase imaging: identification of a signal network that functions independently of ethylene, SA and Me-JA but is dependent on MAPKK activity. Plant J 24:569–582
Gupta AK, Singh Y, Jain AK, Singh D (2014) Prevalence and incidence of bakanae disease of rice in Northern India. J Agric Search 1(4):233–237
Horan PK, Kappler JW (1977) Automated fluorescent analysis for cytotoxicity assays. J Immunol Methods 18:309–316
https://worldpopulationreview.com/country-rankings/rice-production-by-country.
Kasana RC, Salwan R, Dhar H, Dutt S, Gulati A (2008) A rapid and easy method for the detection of microbial cellulases on agar plates using Gram’s iodine. Curr Microbiol 57:503–507. https://doi.org/10.1007/s00284-008-9276-8
Kato A, Taiji M, Kana N, Hideaki T, Tohru T (2012) Visualize interactions between the Bakanae disease pathogen Gibberella fujikuroi and the agent Talaromyces sp KNB-422. J Gen Plant Pathol 78(1):54–61
Klein SM, Cohen G, Cederbaum AI (1981) Production of formaldehyde during metabolism of dimethyl sulfoxide by hydroxyl radical-generating systems. Biochemistry 20(21):6006–6012
Knudsen IMB, Hockenhull J, Jensen DF, Gerhardson B, Hökeberg M, Tahvonen R, Teperi E, Sundheim L, Henriksen B (1997) Selection of biological control agents for controlling soil and seed-borne diseases in the field. Eur J Plant Pathol 103:775–784. https://doi.org/10.1023/A:1008662313042
Krishan A (1975) Rapid flow cytofluorometric analysis of mammalian cell cycle by propidium iodide staining. J Cell Biol 66(1):188–193
Kumar S, Kaushik N, Edrada-Ebel R, Ebel R, Proksch P (2011) Isolation, characterization, and bioactivity of endophytic fungi of Tylophora indica. World J Microbiol Biotechnol 27:571–577. https://doi.org/10.1007/s11274-010-0492-6
Kumar PMK, SiddeGowda DK, Moudgal R, Kumar NK, Pandurange Gowda KT, Vishwanath K (2013) Impact of fungicides on rice production in India. In: Fungicides - Showcases of Integrated Plant Disease Management from around the world, 77–98. (www.creativecommons.org)
Ling KC (1980) Studies on rice diseases. In: Rice Improvement in China and other Asian Countries. International Rice Research Institute and Chinese Academy of Agricultural Science. 135–148
Marcello CM, Steindorff AS, da Silva SP, da Nascimento Silva R, Bataus LAM, Ulhoa CJ (2010) Expression analysis of the exo-β-1, 3-glucanase from the mycoparasitic fungus Trichoderma asperellum. Microbiol Res 165(1):75–81
Marois JJ, Fravel DR, Papavizas GC (1984) Ability of Talaromyces flavus to occupy the rhizosphere. Soil Bio Biochem 16(4):387–390
Montano PF, Alias-Villegas C, Bellogin RA, del-Cerro P, Espuny MR, Jimenez-Guerrero I et al (2014) Plant growth promotion in cereal and leguminous agricultural important plants: from microorganism capacities to crop production. Microbiol Res 169:325–336. https://doi.org/10.1016/j.micres.2013.09.011
Morton DJ, Stroube WH (1955) Antagonistic and stimulating effects of soil microorganisms upon sclerotium. Phytopathol 45:417–420
Murray MG, Thompson WF (1980) Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res 8:4321
Nishiguchi MK, Doukakis P, Egan M, Kizirian D, Phillips A, Prendini L et al (2002) DNA isolation procedures. In: R. DeSalle, G. Giribet, and W. Wheeler (eds) in Techniques in Molecular Systematics and Evolution. Springer Science & Business Media, Basel, 249–287
Ou SH (1985) Rice Diseases. In: 2nd ed. Commonwealth Mycological Institute, Kew, Surrey, England, UK, pp 380
Park WS, Choi HW, Han SS, Shin D, Shim HK, Jung ES, Lee SW, Lim CK, Lee YH (2009) Control of bakanae disease of rice by seed soaking into the mixed solution of prochloraz and fludioxonil. Res Plant Dis 15:94–100. https://doi.org/10.5423/RPD.2009.15.2.094
Radwan DEM, Fayez KA, Mahmoud S, Lu G (2010) Modifications of antioxidant activity and protein composition of bean leaf due to Bean yellow mosaic virus infection and salicylic acid treatments. Acta Physiol Plant 32(5):891–904
Rotman B, Papermaster BW (1966) Membrane properties of living mammalian cells as studied by enzymatic hydrolysis of fluorogenic esters. Proc Natl Acad Sci USA 55:134–141
Schulz B, Boyle C, Draeger S, Römmert AK, Krohn K (2002) Endophytic fungi: a source of novel biologically active secondary metabolites. Mycol Res 106(9):996–1004
Shanmugam V, Kanoujia N (2011) Biological management of vascular wilt of tomato caused by Fusarium oxysporum f.sp. lycopersici by plant growth-promoting rhizobacterial mixture. Bio Control 57:85–93
Shanmugam V, Sharma V, Ananthapadmanaban (2008) Genetic relatedness of Trichoderma isolates antagonistic against Fusarium oxysporum f.sp. dianthi inflicting carnation wilt. Folia Microbiol 53(2):130–138
Shanmugam V, Thakur H, Gupta S (2012) Use of chitinolytic Bacillus atrophaeus strain S2BC-2 antagonistic to Fusarium spp. for control of rhizome rot of ginger. Ann Microbiol 63:989–996
Shanmugam V, Thakur H, Kaur J, Gupta S, Rajkumar S, Dohroo NP (2013) Genetic diversity of Fusarium spp. inciting rhizome rot of ginger and its management by plant growth-promoting rhizobacterial mixtures in the western Himalayas. Bio Control 66:1–7
Sharma V, Shanmugam V (2012) Purification and characterization of an extracellular 24 kDa chitobiosidase from the mycoparasitic fungus Trichoderma saturnisporum. J Basic Microbiol 52:324–331
Singh R, Sunder S (2012) Foot rot and bakanae of rice: an overview. Rev Pl Pathol 5:566–604
Singh A, Singh VK, Singh S, Pandian R, Ellur RK, Singh D, Bhowmick PK et al (2012a) Molecular breeding for the development of multiple disease resistance in Basmati rice. AoB Plants. https://doi.org/10.1093/aobpla/pls029
Singh AK, Singh D, Singh AK, Gade RM, Sangle UR (2012b) Good Agronomic Practices (GAP)-An efficient and eco-friendly tool for sustainable management of plant diseases under changing climate scenario. J Plant Dis Sci 7(1):1–8
Singh AK, Singh A, Singh VK, Gopala KS, Ellur RK, Singh D et al (2014) In: Xie P., Hardy B (eds) in Proceedings of the 6th International Hybrid Rice Symposium, Public private partnership for hybrid rice. International Rice Research Institute, Los Banos, 261–272
Terhonen E, Sipari N, Asiegbu FO (2016) Inhibition of phytopathogens by fungal root endophytes of Norway spruce. Biol Control 99:53–63. https://doi.org/10.1016/j.biocontrol.2016.04.006
Thordal-Christensen H, Zhang Z, Wei Y, Collinge DB (1997) Subcellular localization of H2O2 in plants. H2O2 accumulation in papillae and hypersensitive response during the barley—powdery mildew interaction. Plant J 11(6):1187–1194
Watanabe S, Kumakura K, Izawa N, Nagayama K, Mitachi T, Kanamori M, Arie T (2007) Mode of action of Trichoderma asperellum SKT-1, a biocontrol agent against Gibberella fujikuroi. Pestic Sci 0706230005–0706230005.
White TM, Bruns T, Lee S, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal DNA for phylogenetics. In: Inns MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR protocols: a guide to methods and applications. Academic press, San Diego, pp 315–321
Zheng Y, Xue QY, Xu LL, Xu Q, Lu S, Gu C, Guo JH (2011) A screening strategy of fungal biocontrol agents towards Verticillium wilt of cotton. Biol Control 56:209–216. https://doi.org/10.1016/j.biocontrol.2010.11.010
Acknowledgements
The authors are thankful to the Head, Division of Plant Pathology and Teri Labs, IHC (Indian Habitat Centre) for providing lab facilities and resources for the experiments.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
BMB: study design; NK and SBT: conceived and supervised the study; BMB and NK: provided cultures and lab facilities at IARI-PUSA and Teri labs, IHC; KR and BMB: analyzed the data; KR: wrote the first draft of the manuscript; BMB, NK, SBT: edited the manuscript and reviewed the literature. All authors commented on previous versions of the manuscript and have read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Additional information
Communicated by Erko Stackebrandt.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.


Rights and permissions
About this article
Cite this article
Rawat, K., Tripathi, S.B., Kaushik, N. et al. Management of bakanae disease of rice using biocontrol agents and insights into their biocontrol mechanisms. Arch Microbiol 204, 401 (2022). https://doi.org/10.1007/s00203-022-02999-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00203-022-02999-3