Abstract
Purpose
Clinical examination is often the first step to diagnose shock and estimate cardiac index. In the Simple Intensive Care Studies-I, we assessed the association and diagnostic performance of clinical signs for estimation of cardiac index in critically ill patients.
Methods
In this prospective, single-centre cohort study, we included all acutely ill patients admitted to the ICU and expected to stay > 24 h. We conducted a protocolised clinical examination of 19 clinical signs followed by critical care ultrasonography for cardiac index measurement. Clinical signs were associated with cardiac index and a low cardiac index (< 2.2 L min−1 m2) in multivariable analyses. Diagnostic test accuracies were also assessed.
Results
We included 1075 patients, of whom 783 (73%) had a validated cardiac index measurement. In multivariable regression, respiratory rate, heart rate and rhythm, systolic and diastolic blood pressure, central-to-peripheral temperature difference, and capillary refill time were statistically independently associated with cardiac index, with an overall R2 of 0.30 (98.5% CI 0.25–0.35). A low cardiac index was observed in 280 (36%) patients. Sensitivities and positive and negative predictive values were below 90% for all signs. Specificities above 90% were observed only for 110/280 patients, who had atrial fibrillation, systolic blood pressures < 90 mmHg, altered consciousness, capillary refill times > 4.5 s, or skin mottling over the knee.
Conclusions
Seven out of 19 clinical examination findings were independently associated with cardiac index. For estimation of cardiac index, clinical examination was found to be insufficient in multivariable analyses and in diagnostic accuracy tests. Additional measurements such as critical care ultrasonography remain necessary.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Abnormal clinical signs and a low cardiac index were often present in acutely admitted ICU patients and seven out of nineteen clinical examination findings were independently associated with cardiac index. To estimate cardiac index, clinical examination performs insufficiently in both multivariable analyses and in diagnostic accuracy tests; additional measurements such as critical care ultrasonography remain necessary. |
Introduction
Clinical examination is the first step to estimate cardiac index of critically ill patients to guide interventions and further diagnostic testing. Clinical signs such as altered consciousness, oliguria and a cold, clammy skin indicate organ hypoperfusion and are used to diagnose shock in critically ill patients [1]. If the initial clinical examination is inconclusive, further hemodynamic assessment with critical care ultrasonography (CCUS) is advocated [1, 2]. The evidence base of clinical examination is currently considered ‘best practice’ as there are scarce data on its diagnostic value, especially in comparison with newer, non-invasive bedside tools such as CCUS [1, 3].
Clinical examination for diagnosing shock is fast, easy to conduct and low in cost, yet its diagnostic accuracy is questioned [3,4,5]. Particularly, physicians seem insufficiently capable of diagnosing a low cardiac index purely based on their clinical examination [6,7,8,9]. Previous studies scarcely specified their methods of clinical examination in terms of variables collected and definitions employed, leaving variability at the physician’s discretion and making these studies difficult to reproduce [7, 9,10,11,12,13,14,15]. Cardiac index was measured only in small samples of selected patients who failed to respond to initial therapy or in whom clinical examination alone was deemed insufficient, so the accuracy of clinically estimated cardiac index was biased by definition [1, 12,13,14,15].
The value of clinical signs for estimating cardiac index remains to be established in a large, consecutively recruited cohort of critically ill patients. Our aim was to study the diagnostic performance of clinical examination in a twostep approach: to establish (1) which combination of clinical examination findings are independently associated with cardiac index and (2) the performance of clinical signs to diagnose a low or high cardiac index. The Simple Intensive Care Studies-I (SICS-I) was designed to assess the ability of clinical examination to estimate cardiac index and to identify patients with a low or high cardiac index [16].
Methods
Design, setting and patients
We conducted the prospective, observational, single-centre SICS-I following a pre-published protocol and statistical analysis plan (SAP; clinicaltrials.gov; NCT02912624) [17]. All consecutive patients admitted to the intensive care unit (ICU) of the University Medical Center Groningen (UMCG) between 27 March 2015 and 22 July 2017 were considered eligible. We included patients who were aged 18 years or older, had an unplanned ICU admission and were expected to stay for at least 24 h. We excluded patients if their ICU admission was planned preoperatively, if acquiring research data interfered with clinical care (e.g., mechanical circulatory support) and if informed consent was not provided (e.g., refusal, serious language barrier). In unresponsive patients, informed consent was first obtained from the legal representatives. Consent for use of the study data was asked at a later time if the patient recovered consciousness. If the patient died before consent was obtained, the study data was used, and the legal representatives were informed on the study. The study was approved by the local institutional review board (METc M15.168207).
All patients were included within the first 24 h of their ICU admission and underwent clinical examination, immediately followed by CCUS. Patients were included by medical research interns and PhD students who had received a focused CCUS training course given by experienced cardiologist-intensivists (protocol in Supplements 1) [16]. These researchers were not involved in patient care, and their findings were not revealed to the patients’ caregivers.
Clinical examination
All clinical examinations were standardised and cut-off values for abnormal clinical signs predefined in the protocol (clinicaltrials.gov; NCT02912624). We recorded in total 19 clinical signs per patient (e-Table 1). Respiratory rate, heart rate and rhythm, arterial blood pressures and central venous pressures were recorded from the bedside monitor. Patients were auscultated for the presence of cardiac murmurs and crepitations. Clinical signs reflecting organ perfusion were obtained from the three organs readily accessible to clinical examination: cerebral (mental status), renal (urine output) and skin perfusion (capillary refill time (CRT), central-to-peripheral temperature difference (ΔTc–p) and skin mottling). Mental status was assessed with the AVPU scale, which consists of the categories ‘Alert’, ‘responsive to Voice’, ‘responsive to Pain’ and ‘Unresponsive’ and was not scored in patients who were receiving sedative drugs or who were admitted after a trauma. Urine output was scored 1 and 6 h prior to the clinical examination, adjusted for body weight, and considered decreased if < 0.5 mL kg−1 h−1. CRT was the time for skin colour to fully return after applying firm pressure at the sternum, index finger, and knee for 15 s and considered prolonged if > 4.5 s [18]. ΔTc–p was the difference between central temperature measured by a bladder thermistor catheter and peripheral temperature measured by a skin probe on the big toe and dorsum of the foot and considered abnormal if > 7 °C [19]. The degree of skin mottling was rated at the knee according to a score from 0 to 5, where 0–1 was regarded as mild, 2–3 as moderate and 4–5 as severe mottling [20].
Outcome definition
Cardiac index was measured by transthoracic echocardiography using the Vivid-S6 system (General Electric, Horton, Norway) with cardiac probe M3S or M4S, and with default cardiac imaging setting. The parasternal long axis (PLAX) was used to measure the left ventricular outflow tract (LVOT) diameter. In the apical five chamber (AP5CH) view, a pulse wave Doppler signal in the LVOT was used to measure the velocity time integral (VTI): three VTIs were traced when the heart rhythm was regular and eight VTIs when the heart rhythm was irregular. Cardiac output was calculated using an established formula and was adjusted for body surface area, i.e. cardiac index, because it allowed us to compare patients with different body dimensions [21]. We tested the diagnostic accuracy of clinical signs on four frequently used cut-off values: below 2.2 and 2.5. and above 4.0 and 4.5 L min−1 m−2 [10, 11, 22, 23].
All CCUS images were validated and each cardiac index measured by experts from an independent imaging core laboratory (Groningen Imaging Core Laboratory, www.g-icl.com). These experts were blinded for the clinical examination findings. The most-often used modality for measuring cardiac index in the literature currently is the transpulmonary thermodilution method [24, 25]. We chose CCUS because it is non-invasive, its use is advocated by guidelines, and ensured high-quality measurements throughout validation by experts who followed recent guidelines [26].
Sample size and missing data
No previous research has studied clinical examination in a cohort of consecutively included critically ill patients. Therefore, we estimated our sample size based on the number of acute ICU admissions annually (n = 1500) and estimated that half would fulfil the inclusion criteria. When our sample size exceeded 1000 patients, we calculated a potentially detectable difference using CRT as an example; we were able to detect a 0.10 L min−1 m−2 increase in cardiac index for each second of CRT increase with a power of 100% and alpha of 0.015 [17]. Missing clinical examination values were imputed using multiple imputations (20 times) as these were considered missing at random. Regression coefficients of our final models were averaged using Rubin’s formula [27]. Central venous pressure was missing in 822 (76%) patients and was therefore excluded from the analyses and imputations. Following our SAP, we also imputed missing cardiac index values based on validated LVOT diameters and VTIs (Supplements 2; sensitivity analyses).
Analytical approach
The aims of our analyses were twofold: first, we conducted a least-squares linear regression analysis to identify the clinical examination findings that were independently associated with cardiac index as a continuous variable. Second, we calculated diagnostic test accuracies for each clinical sign and conducted multivariable logistic regression analyses to determine which combined clinical signs were independently associated with a low (< 2.2 and < 2.5 L min−1 m−2) or high (> 4.0 and > 4.5 L min−1 m−2) cardiac index.
Multivariable model development and validation
We conducted a linear regression analysis when using cardiac index as a continuous variable and logistic regression analysis when using a dichotomised cardiac index. We used a p < 0.25 threshold for inclusion in the multivariable models, which was constructed using forward stepwise regression by adding blocks of variables. Noradrenaline infusion rate was added as a confounder to our multivariable models on a theory-driven basis. For the linear regression model, normality of the residuals was assessed with kernel density plots and multicollinearity was checked with variance inflation factors. The model was internally validated with bootstrap sampling, in which we tested whether each predictor was significant in at least 80 of the 100 bootstrap replications. For the logistic regression model, we assessed calibration with the Hosmer–Lemeshow test and evaluated discrimination using receiver operating characteristic (ROC)-curves.
Diagnostic test accuracy
The following diagnostic test accuracies were calculated: sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and overall accuracy. Furthermore, we calculated likelihood ratios (LRs), which represent the increase of probability of a low cardiac index when a clinical sign is abnormal (positive LR) or normal (negative LR) [28]. Following specific Standards for Reporting of Diagnostic Accuracy Studies of medical history and physical examination [29], we summarised all 2 × 2 tables in an overview table (e-Tables 7a and 8a). Clinical signs reflecting central circulation were also dichotomised to test their diagnostic performance.
Subgroup analyses
We conducted two planned subgroup analyses on the diagnostic test accuracies. First, we stratified the study population by noradrenaline administration. Second, we divided patients by primary reasons of ICU admittance as these underlying pathologies may have influenced cardiac index: i.e., acute liver failure or post orthotopic liver transplantation (OLT), heart failure, septic shock, cardiac arrest, and central nervous system (CNS) pathologies.
Statistical significance
We conducted our analyses with Stata version 15.1 software (StataCorp, College Station, TX, USA) and followed a published SAP [17]. Cardiac index was one of the six primary outcomes tested in our cohort and, therefore, we adjusted for multiple hypothesis testing [16, 30]. We refer to our SAP for more details (Supplements 3), but in short a p value of 0.015 indicated statistical significance and p values between 0.015 and 0.05 indicated suggestive significance with an increased family-wise error rate [17]. Accordingly, we presented our primary analysis with 98.5% CI, all secondary analyses with 95% CIs, and discussed any suggestively significant findings based on the p value and results from the bootstrap replications.
Results
Study population
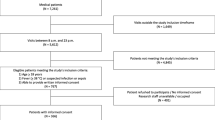
A total of 1212 patients were eligible, of which 137 patients were not included because the desired CCUS window was obstructed by chest drains, wounds, emphysema, or prone positioning (n = 80); routine clinical care such as acute surgery or angiography did not allow patient inclusion (n = 40); or other reasons such as colonisation with multiresistant bacteria (n = 17), leaving 1075 patients for inclusion. Of these, 292 (27%) patients were excluded because one or both of the CCUS views were of insufficient quality as scored by the independent echocardiography core laboratory, resulting in a total of 783 (73%) patients included in the current analyses (Fig. 1). One-third of the patients were admitted after acute or complicated surgery, and the most common admission diagnoses were of cardiovascular or respiratory origin (Table 1). The median time from ICU admission to inclusion was 15 h (IQR 8–20 h).
The mean cardiac index was 2.65 ± 0.93 L min−1 m−2. Table 2 shows that 12 clinical examination findings differed significantly over five cardiac index categories. Clinical signs reflecting normal skin, renal and cerebral perfusion were present in 36 (5%) patients. Abnormal clinical signs were observed in 598 (76%) patients for skin perfusion, 536 (68%) patients for renal perfusion, and 204 (26%) patients for cerebral perfusion. Both cerebral and renal perfusion were abnormal in 141 (18%) patients; skin, renal and cerebral perfusion were abnormal in 103 (13%) patients.
Clinical examination associated with cardiac index
Univariable analyses in the overall population showed that heart rate, systolic blood pressure and all clinical signs reflecting abnormal organ perfusion were statistically significantly associated with cardiac index (supplements; e-Table 2). The strongest association was found for heart rate with an R2 of 0.15. At the mean heart rate of 87 beats per minute, the mean cardiac index was 2.1 L min−1 m−2 with an individual 95% CI that ranged from 0.87 to 4.1 L min−1∙m−2 (e-Fig. 1), reflecting the inaccuracy of a univariable prediction. In multivariable linear regression seven clinical signs, i.e., respiratory rate, heart rate and rhythm, systolic and diastolic blood pressure, ΔTc–p, and CRT were independently associated with cardiac index (e-Table 3). The presence of cardiac murmurs had a p value > 0.015 and was statistically significant in less than 80 of the 100 bootstrap replications. The multivariable model had an R2 of 0.30 (98.5% CI 0.26–0.37). Other model diagnostics and sensitivity analyses are presented in the supplements (e-Figs. 2–4 and e-Tables 4, 5).
Diagnostic test performance of clinical signs
Accuracy of single clinical signs
A cardiac index below 2.2 L min−1 m−2 was observed in 280 (36%) patients. The performance of the 19 clinical signs to diagnose a low cardiac index showed that none had sensitivities, PPVs and NPVs that exceeded 90% (Table 3). A specificity above 90% was found when patients had atrial fibrillation, a systolic blood pressure below 90 mmHg or a diastolic blood pressure below 45 mmHg, an altered consciousness without sedation (i.e., patients who reacted to a pain stimulus only or who were unresponsive), a CRT of > 4.5 s at the sternum, or had mottling over the knee (Fig. 2). One of these five clinical signs was abnormal in 110 (39%) of the 280 patients with a cardiac index below 2.2 L min−1 m−2. A low cardiac index was over 1.5 times more likely when patients had atrial fibrillation, subjectively cold feet, or a CRT at the sternum or index finger of more > 4.5 s (Table 3; positive likelihood ratio).
The diagnostic performance was also assessed for cardiac index cut-off values below 2.5 and above 4.0 and 4.5 L min−1 m−2, and results are presented in the supplements (e-Fig. 6, e-Tables 7 and 8). The accuracy of diagnostic test performance was comparable in all 1075 patients with an imputed cardiac index (e-Tables 9, 10).
Accuracy of combined clinical signs
The PPV of a cardiac index below 2.2 L min−1 m−2 was higher when patients had lower heart rates and lower systolic blood pressures, and when more organs showed signs of hypoperfusion (Fig. 3). Multivariable logistic regression analyses adjusted for noradrenaline infusion rate showed that respiratory rate, heart rate, atrial fibrillation, systolic and diastolic blood pressure, CRT at the sternum and ΔTc–p were independently associated with a cardiac index below 2.2 L min−1 m−2. The model had an area under the ROC of 0.74 (95% CI 0.70–0.78; e-Table 6). These clinical signs correctly classified 556 (71%) of the 783 patients into a low or normal cardiac index.
Positive predictive values of combined clinical signs to diagnose a cardiac index < 2.2 L min−1 m−2. The figure displays the positive predictive value for combinations of the clinical signs heart rate, systolic blood pressure, and number of skin signs showing hypoperfusion (i.e., capillary refill time, cold peripheral temperature, and skin mottling). The figure shows that the PPV increases when patients have lower heart rates combined with lower systolic blood pressures and more signs of skin hypoperfusion. SBP systolic blood pressure
Subgroup analyses
We conducted two predefined subgroup analyses according to noradrenaline use and primary reason for admission. The subgroups were either small (n < 50) or the diagnostic test performance showed little improvement compared to the entire cohort (i.e., < 5%; e-Fig. 7).
Discussion
In the SICS-I, we studied the association of 19 clinical examination findings with cardiac index and assessed their performance in diagnosing a low cardiac index in a consecutive cohort of acutely ill patients admitted to the ICU. We observed that only 5% of the patients had clinical signs indicating normal cerebral, renal and skin perfusion. A little over one-third of all patients had a cardiac index below 2.2 L min−1 m−2 and clinical examination findings were more often abnormal when cardiac index was low. Seven clinical examination findings which reflect respiration, central circulation and skin hypoperfusion were independently associated with cardiac index. Five clinical signs with a high specificity may be used to conclude that a low cardiac index is likely. Nevertheless, the performance of clinical examination in both multivariable analyses and diagnostic tests is insufficient for estimating cardiac index.
This study is the first to associate a broad set of clinical examination variables with cardiac index measured by CCUS. The multivariable regression analyses of our primary outcome showed that 30% of the variance was explained by the model, implicating that clinical examination is insufficiently capable of estimating cardiac index. Compared to smaller studies which assessed one, two or three clinical examination variables, the associations for heart rate, respiratory rate and ΔTc–p gradients were similar [31,32,33,34,35]. In contrast, by including all previously clinical signs, we showed that a CRT measured at the sternum and not peripheral CRT is independently associated with cardiac index [20].
Studies that reported on the physician’s educated guess of cardiac output lacked the design to assess the diagnostic performance of each clinical sign, obtained according to strict definitions. Using multivariable logistic regression, we correctly classified 556 (71%) patients into a low or normal cardiac index, which contrasts with others who found agreements of 50–60% [8,9,10,11, 13,14,15]. One other study evaluated three standardised clinical signs and also found that an abnormality of all three clinical signs have high specificities and low sensitivities to diagnose a low cardiac index [36]. In the above mentioned studies, cardiac index was obtained with transpulmonary thermodilution, compared to echocardiography in our study; evidence indicates that these two techniques may not be interchangeable [37].
Implications for practice
Clinical signs are important warning signals of a deteriorating patient condition, but cannot reliably indicate whether the cardiac index is low, normal or high, or the underlying cause. Our results show both the relevance and limitations of clinical examination: five clinical signs had a specificity above 90%, yet the PPV is much more pertinent than specificity in the clinical process, as it reflects the probability that a low CI is present given that the clinical signs indicate hypoperfusion. The PPV of single clinical signs (Fig. 2) is too low in the majority of patients. PPVs above 90% were observed in a minority of patients with several abnormal clinical signs (Fig. 3). It is unacceptable for a diagnostic test to miss a low cardiac index in one-quarter of cases, or even a larger proportion when diagnosing a high cardiac index (e-Table 6). If physicians immediately perform CCUS, they could diagnose cardiac failure within a few minutes and perform the adequate supportive and therapeutic measures. In the acute setting, this is superior to the collection of urine over 1 h.
The results of our study imply that physicians should not rely solely on clinical signs for their decision making. Although the clinical signs atrial fibrillation, systolic blood pressure below 90 mmHg, altered consciousness, CRT of > 4.5 s at the sternum and skin mottling over the knee make a low cardiac index very likely, we advocate ultrasonography for additional evaluation in these patients. For patients with normal clinical signs additional evaluation should be performed on indication. Therewith, we verify two important statements of the current circulatory shock guidelines for which no evidence base existed (i.e., ‘best practice’) [1].
Strengths and limitations
The clinical examination findings measured in the SICS-I were prone to confounding by interventions. The administration of noradrenaline may have influenced both cardiac index and organ perfusion variables, which is why we added it as a confounder in our multivariable models. The observational design of our study does not allow for conclusions if the measured cardiac index is sufficient for the individual patient. CCUS is focused on obtaining few potentially important measurements and therewith valve pathologies can be overseen. Additional CCUS measures such as ejection fraction and end-diastolic volumes are needed to distinguish between a compensating or a failing heart, but this was beyond the scope of our current research question.
The SICS-I included acutely ill patients admitted to the ICU of a tertiary referral hospital. Compared to others who selected patients with sepsis or myocardial infarction, we studied the value of clinical examination in a large, unselected cohort. Since PPV and NPV values are dependent on the baseline prevalence of low cardiac index, we selected all consecutive patients to maximise representativeness of our cohort to the ICU. However, our study requires external validation before its findings can be applied to other ICUs. Furthermore, our study did not test the inter- and intra-observer agreement of cardiac index measured with CCUS; we will address the inter-observer agreement (researcher versus expert) separately.
Conclusion
Abnormal clinical signs and a low cardiac index were often present in acutely admitted ICU patients, and seven out of 19 clinical examination findings were independently associated with cardiac index. To estimate cardiac index, clinical examination performs insufficiently in both multivariable analyses and in diagnostic accuracy tests. Additional measurements such as critical care ultrasonography remain necessary.
References
Cecconi M, De Backer D, Antonelli M et al (2014) Consensus on circulatory shock and hemodynamic monitoring. Task force of the European Society of Intensive Care Medicine. Intensive Care Med 40:1795–1815. https://doi.org/10.1007/s00134-014-3525-z
Vincent JL, De Backer D (2013) Circulatory shock. N Engl J Med 369:1726–1734. https://doi.org/10.1056/NEJMra1208943
Narula J, Chandrashekhar Y, Braunwald E (2018) Time to add a fifth pillar to bedside physical examination: inspection, palpation, percussion, auscultation, and insonation. JAMA Cardiol. https://doi.org/10.1001/jamacardio.2018.0001
Verghese A, Charlton B, Kassirer JP, Ramsey M, Ioannidis JP (2015) Inadequacies of physical examination as a cause of medical errors and adverse events: a collection of vignettes. Am J Med. https://doi.org/10.1016/j.amjmed.2015.06.004
Elder A, Japp A, Verghese A (2016) How valuable is physical examination of the cardiovascular system? BMJ 354:i3309. https://doi.org/10.1136/bmj.i3309
Hiemstra B, Eck RJ, Keus F, van der Horst ICC (2017) Clinical examination for diagnosing circulatory shock. Curr Opin Crit Care. https://doi.org/10.1097/mcc.0000000000000420
Perel A, Saugel B, Teboul JL et al (2016) The effects of advanced monitoring on hemodynamic management in critically ill patients: a pre and post questionnaire study. J Clin Monit Comput 30:511–518. https://doi.org/10.1007/s10877-015-9811-7
Duan J, Cong LH, Wang H, Zhang Y, Wu XJ, Li G (2014) Clinical evaluation compared to the pulse indicator continuous cardiac output system in the hemodynamic assessment of critically ill patients. Am J Emerg Med 32:629–633. https://doi.org/10.1016/j.ajem.2014.03.023
Nowak RM, Sen A, Garcia AJ, Wilkie H, Yang JJ, Nowak MR, Moyer ML (2012) The inability of emergency physicians to adequately clinically estimate the underlying hemodynamic profiles of acutely ill patients. Am J Emerg Med 30:954–960. https://doi.org/10.1016/j.ajem.2011.05.021
Iregui MG, Prentice D, Sherman G, Schallom L, Sona C, Kollef MH (2003) Physicians’ estimates of cardiac index and intravascular volume based on clinical assessment versus transesophageal Doppler measurements obtained by critical care nurses. Am J Crit Care 12:336–342
Veale WN Jr, Morgan JH, Beatty JS, Sheppard SW, Dalton ML, Van de Water JM (2005) Hemodynamic and pulmonary fluid status in the trauma patient: are we slipping? Am Surg 71:621–626
Connors AF Jr, Dawson NV, McCaffree R, Gray BA, Siciliano CJ (1987) Assessing hemodynamic status in critically ill patients: do physicians use clinical information optimally? J Crit Care 2:174–180
Staudinger T, Locker GJ, Laczika K et al (1998) Diagnostic validity of pulmonary artery catheterization for residents at an intensive care unit. J Trauma 44:902–906
Mimoz O, Rauss A, Rekik N, Brun-Buisson C, Lemaire F, Brochard L (1994) Pulmonary artery catheterization in critically ill patients: a prospective analysis of outcome changes associated with catheter-prompted changes in therapy. Crit Care Med 22:573–579
Steingrub JS, Celoria G, Vickers-Lahti M, Teres D, Bria W (1991) Therapeutic impact of pulmonary artery catheterization in a medical/surgical ICU. Chest 99:1451–1455
Hiemstra B, Eck RJ, Koster G et al (2017) Clinical examination, critical care ultrasonography and outcomes in the critically ill: cohort profile of the simple intensive care studies-I. BMJ Open. https://doi.org/10.1136/bmjopen-2017-017170
Hiemstra B, Nolte IM, Thio CH et al. (2018) Detailed statistical analysis plan of the simple intensive care studies-I (SICS-I). Clinicaltrials.gov: NCT02912624. https://clinicaltrials.gov/ct2/show/NCT02912624. Accessed 3 Apr 2018
Schriger DL, Baraff L (1988) Defining normal capillary refill: variation with age, sex, and temperature. Ann Emerg Med 17:932
Curley FJ, Smyrnios NA (1990) Routine monitoring of critically ill patients. J Intensive Care Med 5:153–174. https://doi.org/10.1177/088506669000500402
Ait-Oufella H, Lemoinne S, Boelle PY et al (2011) Mottling score predicts survival in septic shock. Intensive Care Med 37:801–807. https://doi.org/10.1007/s00134-011-2163-y
Coats AJ (1990) Doppler ultrasonic measurement of cardiac output: reproducibility and validation. Eur Heart J 11(Suppl I):49–61
Rao V, Ivanov J, Weisel RD, Ikonomidis JS, Christakis GT, David TE (1996) Predictors of low cardiac output syndrome after coronary artery bypass. J Thorac Cardiovasc Surg 112:38–51
National Heart L, Wheeler AP, Bernard GR et al (2006) Pulmonary-artery versus central venous catheter to guide treatment of acute lung injury. N Engl J Med 354:2213
Godje O, Hoke K, Goetz AE et al (2002) Reliability of a new algorithm for continuous cardiac output determination by pulse-contour analysis during hemodynamic instability. Crit Care Med 30:52–58
Teboul JL, Saugel B, Cecconi M et al (2016) Less invasive hemodynamic monitoring in critically ill patients. Intensive Care Med 42:1350–1359. https://doi.org/10.1007/s00134-016-4375-7
Lang RM, Badano LP, Mor-Avi V et al (2015) Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 16:233–270. https://doi.org/10.1093/ehjci/jev014
White IR, Royston P, Wood AM (2011) Multiple imputation using chained equations: issues and guidance for practice. Stat Med 30:377–399. https://doi.org/10.1002/sim.4067
Bossuyt PM, Reitsma JB, Bruns DE et al (2015) STARD 2015: an updated list of essential items for reporting diagnostic accuracy studies. BMJ. https://doi.org/10.1136/bmj.h5527
Simel DL, Rennie D, Bossuyt PM (2008) The STARD statement for reporting diagnostic accuracy studies: application to the history and physical examination. J Gen Intern Med. https://doi.org/10.1007/s11606-008-0583-3
Jakobsen JC, Wetterslev J, Winkel P, Lange T, Gluud C (2014) Thresholds for statistical and clinical significance in systematic reviews with meta-analytic methods. BMC Med Res Methodol 14:120. https://doi.org/10.1186/1471-2288-14-120
Joly HR, Weil MH (1969) Temperature of the great toe as an indication of the severity of shock. Circulation 39:131–138
Bailey JM, Levy JH, Kopel MA, Tobia V, Grabenkort WR (1990) Relationship between clinical evaluation of peripheral perfusion and global hemodynamics in adults after cardiac surgery. Crit Care Med 18:1353–1356
Vincent JL, Moraine JJ, van der Linden P (1988) Toe temperature versus transcutaneous oxygen tension monitoring during acute circulatory failure. Intensive Care Med 14:64–68
Schey BM, Williams DY, Bucknall T (2009) Skin temperature as a noninvasive marker of haemodynamic and perfusion status in adult cardiac surgical patients: an observational study. Intensive Crit Care Nurs 25:31–37. https://doi.org/10.1016/j.iccn.2008.05.003
Sasse SA, Chen PA, Mahutte CK (1996) Relationship of changes in cardiac output to changes in heart rate in medical ICU patients. Intensive Care Med 22:409–414
Grissom CK, Morris AH, Lanken PN et al (2009) Association of physical examination with pulmonary artery catheter parameters in acute lung injury. Crit Care Med 37:2720–2726
Wetterslev M, Moller-Sorensen H, Johansen RR, Perner A (2016) Systematic review of cardiac output measurements by echocardiography vs. thermodilution: the techniques are not interchangeable. Intensive Care Med 42:1223–1233. https://doi.org/10.1007/s00134-016-4258-y
Acknowledgements
We would like to thank all medical students and coordinators from the SICS Study Group for their devoted involvement with patient inclusions.
SICS Study Group information: The SICS Study Group members include the following: project leaders: Geert Koster, MD; Frederik Keus, MD, PhD; Iwan CC van der Horst, MD, PhD. Research coordinator: Willem Dieperink, PhD. Researchers who conducted patient inclusions: Roos Bleijendaal, MD; Yasmin F. Cawale, MD; Ramon P. Clement, MD; Devon Dijkhuizen, BSc; Ruben J Eck, MD; Bart Hiemstra, MD; Anja Haker, BSc; Casper D.H. Hilbink, MD; Thomas Kaufmann, MD; Martiene Klasen; MD, Manon Klaver, MD; Laura J. Schokking, BSc; Victor W. Sikkens, MD; Madelon Vos, MD; Justin Woerlee, MD and Renske Wiersema, BSc.
Funding
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Author information
Authors and Affiliations
Consortia
Contributions
BH, IvdH and EK drafted the manuscript and conducted the analyses. IvdH and EK were involved in the study conception. BH, RJE and GK developed the protocol and implemented the study. RW, YMH and TK contributed substantially to the data collection. JW and HS contributed to the design of the detailed statistical analysis plan. TWLS, AP, and AMdS critically reviewed the manuscript for important intellectual content. All authors critically reviewed the manuscript and agreed with the final version and findings.
Corresponding author
Ethics declarations
Conflicts of interest
TWLS reports personal fees and other compensation from Edwards Lifesciences, personal fees from Masimo Inc., outside the submitted work. The other authors declare that they have no conflict of interest.
Ethical approval
The study was approved by the local institutional review board (Medisch Ethische Toetsingscommissie, University Medical Center Groningen; METc M15.168207).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
OpenAccess This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Hiemstra, B., Koster, G., Wiersema, R. et al. The diagnostic accuracy of clinical examination for estimating cardiac index in critically ill patients: the Simple Intensive Care Studies-I. Intensive Care Med 45, 190–200 (2019). https://doi.org/10.1007/s00134-019-05527-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-019-05527-y