Abstract
Purpose
The soluble receptor for advanced glycation end-products (sRAGE) is a marker of lung epithelial injury and alveolar fluid clearance (AFC), with promising values for assessing prognosis and lung injury severity in acute respiratory distress syndrome (ARDS). Because AFC is impaired in most patients with ARDS and is associated with higher mortality, we hypothesized that baseline plasma sRAGE would predict mortality, independently of two key mediators of ventilator-induced lung injury.
Methods
We conducted a meta-analysis of individual data from 746 patients enrolled in eight prospective randomized and observational studies in which plasma sRAGE was measured in ARDS articles published through March 2016. The primary outcome was 90-day mortality. Using multivariate and mediation analyses, we tested the association between baseline plasma sRAGE and mortality, independently of driving pressure and tidal volume.
Results
Higher baseline plasma sRAGE [odds ratio (OR) for each one-log increment, 1.18; 95% confidence interval (CI) 1.01–1.38; P = 0.04], driving pressure (OR for each one-point increment, 1.04; 95% CI 1.02–1.07; P = 0.002), and tidal volume (OR for each one-log increment, 1.98; 95% CI 1.07–3.64; P = 0.03) were independently associated with higher 90-day mortality in multivariate analysis. Baseline plasma sRAGE mediated a small fraction of the effect of higher ΔP on mortality but not that of higher VT.
Conclusions
Higher baseline plasma sRAGE was associated with higher 90-day mortality in patients with ARDS, independently of driving pressure and tidal volume, thus reinforcing the likely contribution of alveolar epithelial injury as an important prognostic factor in ARDS. Registration: PROSPERO (ID: CRD42018100241).
Similar content being viewed by others
Because alveolar fluid clearance (AFC) is impaired in most patients with acute respiratory distress syndrome (ARDS) and is associated with higher mortality, we hypothesized that baseline plasma soluble receptor for advanced glycation end-products (sRAGE), a marker of lung epithelial injury and of impaired AFC, would predict mortality, independently of two key mediators of ventilator-induced lung injury such as driving pressure and tidal volume. We conducted a meta-analysis of individual data from 746 patients enrolled in eight prospective randomized and observational studies, and found that higher baseline plasma sRAGE was associated with higher 90-day mortality in patients with ARDS, independently of driving pressure and tidal volume, thus reinforcing the likely contribution of alveolar epithelial injury as an important prognostic factor in ARDS. |
Introduction
The acute respiratory distress syndrome (ARDS) is a clinical syndrome associated with diffuse alveolar injury leading to increased permeability pulmonary edema, alveolar filling, and rapid onset of hypoxemic respiratory failure [1]. Despite improvements in intensive care during the last 15 years, ARDS is still an unrecognized, morbid, and life-threatening condition, with mortality rates of 30–50% [2]. The identification of predictors of poor outcomes and a better understanding of ARDS pathophysiology are warranted to provide further insight into the response to therapeutic strategies and ultimately to improve outcomes of patients with ARDS [3].
The integrity of the alveolar-capillary barrier is necessary for normal pulmonary function, and impaired alveolar fluid clearance (AFC) is a central feature of the pathogenesis of ARDS [4, 5]. The magnitude of damage to the alveolar type (AT) 1 cell could therefore be a major determinant of the severity of ARDS and of its clinical outcomes [6,7,8]. Growing evidence supports a pivotal role for RAGE, the receptor for advanced glycation end-products, in ARDS pathophysiology through the initiation and perpetuation of inflammatory and immune responses [9]. sRAGE, the main soluble form of RAGE, has the most features of a biomarker of lung epithelial injury that could be used in clinical medicine [10], with values for ARDS diagnosis [6, 11, 12], assessment of lung injury severity and impaired AFC [6,7,8, 11, 13, 14], monitoring the response to therapy [15], and identifying subgroups (or subphenotypes) of patients that might benefit from tailored therapy [11, 14, 16]. Notably, recent evidence supports a prognostic value for circulating sRAGE in patients with ARDS; elevated baseline levels of plasma sRAGE are associated with higher mortality in patients receiving high-tidal-volume (VT) ventilation [7], and lower VT ventilation may accelerate the decline in sRAGE levels over the first days of ARDS [11].
In patients with ARDS, the proportion of lung available for ventilation is markedly decreased, reflected in part by a lower respiratory system compliance (CRS) [17]. Normalizing VT to CRS and using this ratio, termed driving pressure (ΔP = VT/CRS), as an index indicating the functional size of the lung provided a better predictor of outcomes in patients with ARDS than VT alone in a recent secondary data analysis [18]. Because higher ΔP may contribute to lung epithelial injury in a rat model of sepsis-induced ARDS [19], we hypothesized that risk stratification provided by ΔP in ARDS [18] could be mediated, at least in part, by the concurrent degree of lung epithelial injury, as assessed by plasma sRAGE [6, 8]. To test the extent to which baseline plasma sRAGE could be associated with higher mortality in ARDS, independent of ΔP and VT, we therefore combined individual patient data from previously published studies of plasma levels of sRAGE during ARDS that included mortality assessment and used both a standard risk analysis with multivariate adjustments and a multilevel mediation analysis [18, 20].
Some of the results of this study have been previously reported in the form of an abstract or oral communication during the American Thoracic Society International Conference (2018).
Methods
Study selection and data collection
Individual participant data were sought from investigators of all prospective clinical studies identified through systematic searches of the published literature using MEDLINE and Web of Science databases (search terms “acute respiratory distress syndrome” and “receptor for advanced glycation end-products” up to March 2016) and by extensive discussions with the investigators (referred to herein as collaborators). Cohort studies, either interventional or observational, were eligible if the following variables were available in adult patients with ARDS: baseline plasma levels of sRAGE, baseline ΔP, tidal volume, and mortality at day 90. Data from each study were obtained using a standardized spreadsheet (appendix); raw data were examined, and inconsistencies or irregularities were clarified with the relevant investigators. This study was exempt from institutional review board approval by the Clermont-Ferrand Sud-Est VI ethics committee because studies that were included were already published and had each previously received local institutional review board approvals and consent from participants. This study was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses of Individual Participants Data (PRISMA-IPD) guidelines [21] (checklist available in the appendix). The protocol was registered on PROSPERO (ID: CRD42018100241) in June 2018.
When available, data were collected on medical history and coexisting conditions (including diabetes, hypertension, dyslipidemia, chronic obstructive pulmonary disease, tobacco smoking, chronic alcohol use, chronic dialysis for end-stage renal disease, hematologic neoplasms, cancer, atherosclerosis, liver cirrhosis), primary ARDS risk factor, severity scores at baseline (Acute Physiology and Chronic Health Evaluation II [22], Acute Physiology and Chronic Health Evaluation III [23], Sequential Organ Failure Assessment [24]), the need for epinephrine, norepinephrine, or dobutamine support, baseline serum creatinine, bilirubin, and sodium and bicarbonate levels.
Information on baseline lung injury severity (ratio of the partial pressure of arterial oxygen to the fraction of inspired oxygen (PaO2/FiO2), severity of ARDS based on the Berlin definition: mild, moderate, or severe), respiratory parameters [VT in ml.kg–1 of predicted body weight (PBW), inspiratory plateau pressure (Pplat), positive end-expiratory pressure (PEEP), and ΔP] and 90-day mortality was provided by collaborators in the greatest detail available. In all of the included studies, baseline levels of plasma sRAGE were measured in duplicate using commercially available enzyme-linked immunosorbent assays (R&D Systems, Minneapolis, MN, USA) at study entry. Apart from plasma sRAGE, all other data from randomized trials were collected after randomization.
Prior to the transformation of the data from each study to a standard format for incorporation into a central database, the data were checked for consistency by a panel of investigators (RB, BP, JMC, and MJ), and any queries were referred back to the collaborators prior to the final harmonization of the data.
Independent variables and outcomes
The primary outcome (the dependent variable) was mortality at 90 days. The independent variables tested as predictors included characteristics of patients (e.g., age), baseline severity of illness (e.g., risk according to SOFA, APACHE II, or III scores, PaO2/FiO2), ventilation variables (e.g., VT, PEEP, ΔP), baseline levels of plasma sRAGE (defined a priori as the primary predictor), and primary ARDS risk factors (e.g., sepsis, pneumonia, severe trauma).
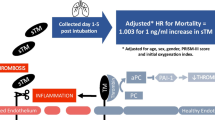
A conceptual diagram of the main objectives of the study is provided in the appendix (Supplementary Fig. 5). The first step was to test the association between higher ΔP and mortality; then, we investigated the association between higher degrees of lung epithelial injury (as assessed by higher baseline plasma sRAGE) and mortality. Finally, to reinforce the independent association between higher baseline plasma sRAGE and higher mortality, mediation analysis was done to assess whether higher degrees of lung epithelial injury (as assessed by baseline plasma sRAGE) might mediate, at least in part, the effects of higher ΔP on mortality. The same approaches were used for the effects of another key mediator of VILI (higher VT) and for the effects of lower PaO2/FiO2 on mortality, a parameter that is frequently used to define ARDS severity [1].
Statistical analysis
Additional details are provided in the appendix. All analyses were performed using Stata software (version 14, StataCorp, College Station, TX) with a two-sided type I error of α = 5%. Comparisons of patient characteristics between survivors and non-survivors were performed using the chi-squared or Fisher’s exact tests for categorical variables, and Student's t test or Mann-Whitney test was used when the assumption of the t test was not met (normality and homoscedasticity studied using the Fisher-Snedecor test) for quantitative variables. Because the available severity scores (SOFA, APACHE II, APACHE III) differed among the studies included in this meta-analysis and because incorporating each of them as a covariate would have led to a reduced number of cases available for multivariate analyses, a risk score was calculated using an average z score as a composite of available scores based on the mean of the standardized variables (by subtracting the mean and then dividing by their standard error) [25]. Mixed logistic regression models were used in univariate analyses and to study the predictive factors in multivariate situations by backward and forward stepwise regression, according to univariate results and to clinical relevance [26, 27]. For analyses of sRAGE levels, PaO2/FiO2, Vt, and PEEP, logarithmic transformation was applied to achieve normal distribution. The study effect was taken into account as a random effect. The interactions between possible predictive factors were also tested. The multicollinearity was studied using usual statistical tests.
To investigate whether baseline plasma sRAGE is more than a baseline risk predictor and to assess the respective contributions of baseline plasma sRAGE, VT, and ΔP for prognosis, we conducted a mediation analysis. When mediation analysis is applied, the goal is to determine whether a specific variable (the “mediator”) has an effect on outcome that explains, in whole or in part, the prognostic effects resulting from another independent variable [20, 28]. A mediation proportion was estimated, indicating how much of the whole prognostic value provided by an independent variable can be explained by the indirect path in which changes in this independent variable drives a change in the mediator, and changes in the mediator then affect outcome (Supplementary Fig. 5 of the appendix). An average causal mediation effect (ACME) was calculated, which express the independent hazard associated with this indirect path [20]. The exposure-mediator interaction effect was tested.
A total of 4.5% data (out of 65,655 data points) were missing. However, no data were missing for the primary outcome. We performed multiple imputation of missing data (missing completely at random) for multivariate analysis, and this did not modify our results. A sensitivity analysis was performed to compare main baseline characteristics and clinical outcomes between patients from studies with plasma sRAGE and ΔP available at baseline (n = 746) and those with either plasma sRAGE or ΔP unavailable at baseline (n = 517).
Results
Data synthesis and patient characteristics
Our database search retrieved 23 articles that were scrutinized with a full text review. In total, eight prospective studies fulfilled our eligibility criteria and were finally included [7, 11, 12, 14, 29,30,31,32] (Fig. 1). This analysis included 746 participants (among whom 700 were complete cases available for multivariate analysis) with available individual records, plasma levels of sRAGE, and follow-up data: 6 were observational studies [11, 12, 14, 29,30,31] and 2 were analyses of saved samples and on-study variables from ARDS patients enrolled in multicenter randomized controlled trials (RCTs) comparing lower and higher VT ventilation [7, 32]. The intraclass correlation coefficient associated with the study effect was equal to 0.03, thus reflecting a minimal study effect in this meta-analysis. Baseline characteristics and main outcomes are reported in Table 1. Data on age, sex, primary ARDS risk factor, baseline PaO2/FiO2, ARDS severity (mild, moderate, or severe), VT, PEEP, baseline plasma sRAGE, and ΔP were available for all 746 patients. Comparisons of baseline characteristics and main clinical outcomes among patients from studies with plasma sRAGE and ΔP available at baseline (n = 746) and those with either plasma sRAGE or ΔP unavailable at baseline (n = 517) are summarized in Table 1 of the appendix.
Association of baseline plasma of sRAGE and driving pressure with 90-day mortality
Baseline levels of plasma sRAGE in patients from each study are summarized in Table 2 of the appendix. Non-survivors at day 90 had higher baseline plasma levels of sRAGE, VT, and ΔP than survivors [4335 (1770–9256) vs. 3198 (1554–6009) pg.ml−1, P = 0.002, 8.8 ± 3.0 vs. 8.2 ± 2.7 ml.kg–1 PBW, P = 0.02, and 20.6 ± 7.0 vs. 19.2 ± 6.8 cmH2O, P = 0.02, respectively) (Table 1). Unadjusted analyses tested the relationship between baseline features and 90-day mortality in our cohort; in these analyses, baseline features such as older age, non-trauma-related ARDS, severe ARDS Berlin class, higher APACHE III, SOFA and risk scores, lower PaO2/FiO2, higher VT, inspiratory plateau pressure, ΔP, and plasma sRAGE, lower bicarbonate and arterial pH, and the need for norepinephrine at baseline were all significantly associated with higher 90-day mortality (Table 1). Next, variables that were significant in univariate analyses (but not already included, directly or indirectly, in baseline severity scores) and non-significant but clinically relevant variables were used to compute odds ratios (OR) for death at day 90 using multivariate logistic regression analysis. Higher baseline plasma sRAGE, along with VT and ΔP, were independently associated with higher 90-day mortality (OR for each one-log increment in plasma sRAGE, 1.18, 95% CI 1.01–1.38, OR for each one-log increment in VT, 1.98, 95% CI 1.07–3.64, and OR for each one-point increment in ΔP, 1.04, 95% CI 1.02–1.07, respectively), even after adjustment for severity of illness (risk score), age, baseline PaO2/FiO2, PEEP and sepsis, pneumonia or trauma as primary ARDS risk factors, and study effect (Fig. 2, Table 3 of the appendix).
Forest plot of odds ratios for death at day 90 after multivariate logistic regression in patients with acute respiratory distress syndrome (n = 700). *A risk score was calculated as a composite of available severity scores (SOFA, APACHE II, APACHE III) combined using an average z score. Study effect was taken into account as a random effect covariate. Plasma levels of sRAGE (in pg.ml−1), PaO2/FiO2, tidal volume, and PEEP are natural log-transformed in the model to meet the assumption of linearity with log odds of outcome; the ORs presented here are for each log increase in the level of plasma sRAGE, PaO2/FiO2, tidal volume, and PEEP. APACHE II Acute Physiology and Chronic Health Evaluation II Score, APACHE III Acute Physiology and Chronic Health Evaluation III Score, SOFA Sequential Organ Failure Assessment Score, ΔP driving pressure, PEEP positive end-expiratory pressure, sRAGE soluble receptor for advanced glycation end-products
Mediation analysis
After observing that ΔP and baseline plasma sRAGE were associated with 90-day mortality, and because there was no exposure-mediator interaction (P = 0.12), we performed a multilevel mediation analysis [33] using study effect as a fixed effect. Increases in both ΔP and (log-transformed) plasma sRAGE were significantly associated with higher mortality in our cohort (step 1, 2 of mediation analysis) (Fig. 3), independently of baseline characteristics, study effect, and severity (OR, 1.05; 95% CI 1.02–1.08 and 1.22; 95% CI 1.04–1.42, respectively). Plasma sRAGE was then tested as a mediator of the effects of ΔP on mortality. The direct association between ΔP and mortality remained significant, and baseline plasma sRAGE mediated 9% (ACME) of the effects of ΔP on mortality. Next, we performed multilevel mediation analysis with Vt and baseline plasma sRAGE and found that the effect of higher VT on mortality was not mediated by plasma sRAGE (Fig. 4). Finally, we performed multilevel mediation analysis with PaO2/FiO2 and baseline plasma sRAGE and found that the effect of lower PaO2/FiO2 on mortality was not mediated by plasma sRAGE (Supplementary Fig. 6 of the appendix).
Mediation analysis of 90-day mortality in patients with acute respiratory distress syndrome. Tested mediator: changes in baseline plasma sRAGE. Independent variable: changes in baseline ΔP. Top: the first step in our mediational analysis was the demonstration that higher ΔP had a measurable impact on mortality after accounting for baseline risk covariates. Middle: second, we checked if mediator changes correlated with higher mortality, after accounting for baseline risk covariates. Bottom: finally, a multilinear regression (mixed effects) calculated the influence of higher ΔP on the tested mediator (baseline plasma sRAGE). Subsequently, we jointly calculated the influence of the mediator on 90-day mortality, after accounting for baseline risk covariates, and the direct effects of the independent variable (higher ΔP). This last step shows that higher plasma sRAGE partially mediates [9%, P = 0.04 for the average causal mediation effect (ACME)] the original effect of baseline ΔP on mortality and, consequently, baseline ΔP remains directly associated with mortality in an independent manner (characterizing incomplete mediation). Mediator and independent variables are assessed as continuous variables. Plasma levels of sRAGE (in pg.ml−1), PaO2/FiO2, tidal volume, and PEEP are natural log-transformed in the model to meet assumption of linearity with log odds of outcome. ARDS acute respiratory distress syndrome, sRAGE soluble receptor for advanced glycation end-products, ΔP driving pressure, PEEP positive end-expiratory pressure
Mediation analysis of 90-day mortality in patients with acute respiratory distress syndrome. Tested mediator: changes in baseline plasma sRAGE. Independent variable: changes in tidal volume. Top: the first step in our mediational analysis was the demonstration that higher tidal volume had a measurable impact on mortality, after accounting for baseline risk covariates. Middle: second, we checked if mediator changes (higher baseline plasma sRAGE) correlated with higher mortality after accounting for baseline risk covariates. Bottom: finally, a multilinear regression (mixed effects) calculated the influence of higher tidal volume on the tested mediator (baseline plasma sRAGE). Subsequently, we jointly calculated the influence of the mediator on 90-day mortality, after accounting for baseline risk covariates, and the direct effects of the independent variable (higher tidal volume). This last step shows that higher plasma sRAGE does not significantly mediate [P = 0.5 for the average causal mediation effect (ACME)] the original effect of higher tidal volume, and, consequently, higher tidal volumes remain directly associated with mortality in an independent manner (characterizing lack of mediation). Mediator and independent variables are assessed as continuous variables. Plasma levels of sRAGE (in pg.ml−1), PaO2/FiO2, tidal volume, and PEEP are natural log-transformed in the model to meet the assumption of linearity with log odds of outcome. PBW predicted body weight, ARDS acute respiratory distress syndrome, sRAGE soluble receptor for advanced glycation end-products, PEEP positive end-expiratory pressure
Discussion
Using a meta-analysis of individual patient data to investigate the relationships between baseline plasma sRAGE, ΔP, VT, and 90-day mortality, our findings indicate that higher plasma levels of sRAGE are associated with higher mortality in ARDS, independent of ΔP and VT. In addition, baseline plasma sRAGE mediated a small fraction of the effect of higher ΔP on mortality, but not those of higher VT or of lower PaO2/FiO2, thus emphasizing the independent prognostic value of plasma sRAGE in patients with ARDS.
The results of this analysis are in agreement with previous recent studies of ΔP in patients with ARDS [2, 18]. In a secondary analysis of trials of mechanical ventilation involving patients with ARDS, in which VT and PEEP were included as independent variables, the dependent variable ΔP was most strongly associated with survival and best stratified risk during ARDS [18]. In this analysis of 3562 patients with ARDS enrolled in 9 previously reported randomized trials, individual changes in VT or PEEP after randomization were not independently associated with survival, and a 1 SD increment in ΔP (approximately 7 cmH2O) was associated with increased mortality (relative risk, 1.41; 95% CI 1.31–1.51; P < 10−3), even in patients receiving protective plateau pressures and VT (relative risk, 1.36; 95% CI 1.17–1.58; P < 10−3) [18]. Indeed, changes in VT or PEEP were associated with survival only if they were among the changes that led to reductions in ΔP (mediation effects of ΔP, P = 0.004 and P = 0.001, respectively) [18]. In the current analysis, PEEP was neither tested as an independent nor as a mediator variable because higher PEEP levels were not associated with mortality in multivariate analysis. The findings supporting an association between elevated baseline ΔP and higher mortality were recently confirmed by both the large multicenter observational LUNG SAFE study [2] and secondary analyses of the PROSEVA and ACURASYS studies [34]. On the other hand, the association with ΔP and mortality was less obvious in the recent ART trial [35]. Interestingly, high intraoperative ΔP and changes in the level of PEEP that resulted in an increase in ΔP were also associated with more postoperative pulmonary complications in at-risk patients having surgery [36].
There is growing evidence supporting a prognostic value for circulating sRAGE in patients with ARDS. Higher baseline plasma sRAGE was associated with mortality in patients receiving high VT ventilation in a retrospective analysis of data and samples from a large RCT of lower VT in ARDS [7], and lower tidal VT may amplify the decline in plasma sRAGE over the first 3 days of ARDS in a small single-center observational study [11], suggesting that ventilation with low VT may cause less injury to the alveolar epithelium, in particular to AT 1 cells, compared with higher VT ventilation. Recently, lower baseline plasma sRAGE was also significantly associated with better outcome in ARDS patients ventilated with low VT and enrolled in a large multicenter observational study [14]. In addition, plasma sRAGE was higher in patients with a hyperinflammatory endotype than in those with a hypoinflammatory endotype, i.e., ARDS subphenotypes with distinct natural histories, clinical and biologic characteristics, clinical outcomes, and therapeutic responses, e.g., to the PEEP level [37] or fluid strategies [38].
In this meta-analysis, we found that baseline plasma sRAGE mediated a small fraction (9%) of the effects of higher ΔP on mortality, independently of ventilator settings (e.g., VT and PEEP), severity of illness, and patient characteristics or coexisting conditions. The factors contributing to the bigger fraction (91%) of the effects of higher ΔP on mortality remain undetermined and may combine both some ventilator settings that contribute to ventilator-induced lung injury and more patient-related variables such as the degree of lung injury and of altered compliance of the respiratory system. The association of high plasma sRAGE and higher ΔP strongly correlates with the highest mortality, thus possibly reinforcing the contributions of lung epithelial injury and impaired AFC [6, 8, 39] as major prognostic factors in ARDS [5, 40, 41].
Although additive and reciprocal effects of both epithelial injury and higher ΔP on mortality may exist, further mechanistic studies are needed to better understand both the implications of the RAGE pathway on lung injury severity (i.e., altered compliance, impaired AFC, and alveolar integrity) [6, 8, 39, 42,43,44,45,46] and the mechanotransduction response of lung alveolar epithelium to ΔP in ARDS [19, 47, 48].
This study has some limitations. First, it included patients from only eight studies, including both observational studies (n = 6) and RCTs (n = 2), despite rigorous and exhaustive literature research. Therefore, our results may require validation in larger cohorts of patients, and high ΔP values in this study may be, at least partially, explained by the use of a large VT in patients enrolled in a historical RCT [7]. All selected studies were prospective, and data from a total of 1107 patients were screened, from which 746 patients had full data for major end points (plasma sRAGE, ΔP, and 90-day mortality) and 700 patients were considered complete cases for multivariate analysis. In addition, such a meta-analysis necessarily may carry some degree of selection bias (such as reflected by a relatively low rate of primary ARDS and some imbalances in prognostic variables in the selected population) and inter-study heterogeneity (intraclass correlation coefficient of 0.03), in part because analysis of possible classifying variables was restricted to the data obtained in the original studies. For example, data on another prognostic factor such as deadspace fraction [49] were unavailable. However, this study provides characterization of the prognostic value of a novel biomarker of lung epithelial injury in the largest cohort of ARDS patients with available data on both ΔP and plasma sRAGE to date. In addition, given the wide time period spanning patient inclusion in individual studies, some important changes in patient management may influence our findings. Second, our analysis does not account for baseline chest wall elastance, although the cyclic gradient of pressures across the lung (that may generate parenchymal injury during ventilation in ARDS) might be lower in patients with increased chest wall elastance, such as in obese patients [47]. However, the associations between ΔP and mortality in ARDS [2], and between ΔP and postoperative pulmonary complications in patients having surgery [36], have been recently confirmed without considering chest wall elastance as a covariate. Third, our conclusions on ΔP are only valid for ventilation in which the patient is not making respiratory efforts because it is difficult to interpret ΔP in actively breathing patients. Fourth, because plasma sRAGE was measured at study entry in all studies and ventilatory variables were collected after randomization in randomized trials, changes in ΔP due to randomization may have moderately biased mediation analysis. Finally, our analysis does not account for changes over time in variables such as plasma sRAGE or ΔP, and the value of such changes to enrich the prognosis in ARDS remains unknown.
This study also has several strengths. First, analyses of individual participant data support the generalizability of our findings, with the usual caveats regarding retrospective analyses of prospectively acquired data. Second, this meta-analysis provides novel and unique findings that further support a prognostic value for plasma sRAGE in ARDS, thus contributing to the characterization of plasma sRAGE as a validated biomarker in patients with the syndrome [6,7,8, 11, 12, 15]. Finally, the use of logistic regression multivariate models and mediation analyses both support baseline plasma sRAGE as a variable that stratified risk, independently of ΔP, VT, and the severity of hypoxemia, thus reinforcing the value of sRAGE as a reliable prognostic marker in ARDS.
In conclusion, these findings provide evidence that alveolar epithelial injury at baseline, as assessed by plasma sRAGE, is an independent variable associated with 90-day mortality in ARDS, independently of ΔP and VT. Although these findings reinforce the likely contribution of alveolar epithelial injury as an important prognostic factor in ARDS, the causal—if not reciprocal—relationship between lung epithelial injury (i.e., higher plasma sRAGE) and higher ΔP deserves further investigation.
References
Definition Task Force ARDS, Ranieri VM, Rubenfeld GD et al (2012) Acute respiratory distress syndrome: the Berlin definition. JAMA 307:2526–2533
Bellani G, Laffey JG, Pham T et al (2016) Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA 315:788–800
Matthay MA, Zimmerman GA, Esmon C et al (2003) Future research directions in acute lung injury: summary of a National Heart, Lung, and Blood Institute working group. Am J Respir Crit Care Med 167:1027–1035
Matthay MA, Wiener-Kronish JP (1990) Intact epithelial barrier function is critical for the resolution of alveolar edema in humans. Am Rev Respir Dis 142:1250–1257
Matthay MA (2014) Resolution of pulmonary edema. Thirty years of progress. Am J Respir Crit Care Med 189:1301–1308
Uchida T, Shirasawa M, Ware LB et al (2006) Receptor for advanced glycation end-products is a marker of type I cell injury in acute lung injury. Am J Respir Crit Care Med 173:1008–1015
Calfee CS, Ware LB, Eisner MD et al (2008) Plasma receptor for advanced glycation end products and clinical outcomes in acute lung injury. Thorax 63:1083–1089
Jabaudon M, Blondonnet R, Roszyk L et al (2015) Soluble receptor for advanced glycation end-products predicts impaired alveolar fluid clearance in acute respiratory distress syndrome. Am J Respir Crit Care Med 192:191–199
Bierhaus A, Humpert PM, Morcos M et al (2005) Understanding RAGE, the receptor for advanced glycation end products. J Mol Med 83:876–886
Ray P, Le Manach Y, Riou B, Houle TT (2010) Statistical evaluation of a biomarker. Anesthesiology 112:1023–1040
Jabaudon M, Futier E, Roszyk L et al (2011) Soluble form of the receptor for advanced glycation end products is a marker of acute lung injury but not of severe sepsis in critically ill patients. Crit Care Med 39:480–488
Jabaudon M, Blondonnet R, Roszyk L et al (2015) Soluble forms and ligands of the receptor for advanced glycation end-products in patients with acute respiratory distress syndrome: an observational prospective study. PLoS One 10:e0135857
Calfee CS, Budev MM, Matthay MA et al (2007) Plasma receptor for advanced glycation end-products predicts duration of ICU stay and mechanical ventilation in patients after lung transplantation. J Heart Lung Transpl 26:675–680
Mrozek S, Jabaudon M, Jaber S et al (2016) Elevated plasma levels of sRAGE are associated with nonfocal CT-based lung imaging in patients with ARDS: a prospective multicenter study. Chest 150:998–1007
Jabaudon M, Hamroun N, Roszyk L et al (2015) Effects of a recruitment maneuver on plasma levels of soluble RAGE in patients with diffuse acute respiratory distress syndrome: a prospective randomized crossover study. Intensive Care Med 41:846–855
Jabaudon M, Blondonnet R, Lutz J et al (2016) Net alveolar fluid clearance is associated with lung morphology phenotypes in acute respiratory distress syndrome. Anaesth Crit Care Pain Med 35:81–86
Gattinoni L, Pesenti A (2005) The concept of “baby lung”. Intensive Care Med 31:776–784
Amato MBP, Meade MO, Slutsky AS et al (2015) Driving pressure and survival in the acute respiratory distress syndrome. N Engl J Med 372:747–755
Samary CS, Santos RS, Santos CL et al (2015) Biological impact of transpulmonary driving pressure in experimental acute respiratory distress syndrome. Anesthesiology 123:423–433
Imai K, Keele L, Tingley D, Yamamoto T (2011) Unpacking the black box of causality: learning about causal mechanisms from experimental and observational studies. Am Polit Sci Rev 105:765–789
Stewart LA, Clarke M, Rovers M et al (2015) Preferred reporting items for a systematic review and meta-analysis of individual participant data: the PRISMA-IPD Statement. JAMA 313:1657–1665
Knaus WA, Draper EA, Wagner DP, Zimmerman JE (1985) APACHE II: a severity of disease classification system. Crit Care Med 13:818–829
Knaus WA, Wagner DP, Draper EA et al (1991) The APACHE III prognostic system. Risk prediction of hospital mortality for critically ill hospitalized adults. Chest 100:1619–1636
Vincent JL, Moreno R, Takala J et al (1996) The SOFA (sepsis-related organ failure assessment) score to describe organ dysfunction/failure. on behalf of the working group on sepsis-related problems of the European Society of Intensive Care Medicine. Intensive Care Med 22:707–710
O’Brien PC (1984) Procedures for comparing samples with multiple endpoints. Biometrics 40:1079–1087
Harrell FE Jr, Lee KL, Mark DB (1996) Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med 15:361–387
Malek MH, Berger DE, Coburn JW (2007) On the inappropriateness of stepwise regression analysis for model building and testing. Eur J Appl Physiol 101:263–264 (author reply 265–6)
Shrout PE, Bolger N (2002) Mediation in experimental and nonexperimental studies: new procedures and recommendations. Psychol Methods 7:422–445
Cartin-Ceba R, Hubmayr RD, Qin R et al (2015) Predictive value of plasma biomarkers for mortality and organ failure development in patients with acute respiratory distress syndrome. J Crit Care 30(219):e1–e7
Brodska H, Malickova K, Valenta J et al (2013) Soluble receptor for advanced glycation end products predicts 28-day mortality in critically ill patients with sepsis. Scand J Clin Lab Invest 73:650–660
Mauri T, Masson S, Pradella A et al (2010) Elevated plasma and alveolar levels of soluble receptor for advanced glycation end products are associated with severity of lung dysfunction in ARDS patients. Tohoku J Exp Med 222:105–112
Determann RM, Royakkers AANM, Haitsma JJ et al (2010) Plasma levels of surfactant protein D and KL-6 for evaluation of lung injury in critically ill mechanically ventilated patients. BMC Pulm Med 10:6
Krull JL, MacKinnon DP (2001) Multilevel modeling of individual and group level mediated effects. Multivar Behav Res 36:249–277
Guérin C, Papazian L, Reignier J et al (2016) Effect of driving pressure on mortality in ARDS patients during lung protective mechanical ventilation in two randomized controlled trials. Crit Care 20:384
Writing Group for the Alveolar Recruitment for Acute Respiratory Distress Syndrome Trial (ART) Investigators, Cavalcanti AB, Suzumura ÉA et al (2017) Effect of lung recruitment and titrated positive end-expiratory pressure (PEEP) vs Low PEEP on mortality in patients with acute respiratory distress syndrome: a randomized clinical trial. JAMA 318:1335–1345
Neto AS, Hemmes SNT, Barbas CSV et al (2016) Association between driving pressure and development of postoperative pulmonary complications in patients undergoing mechanical ventilation for general anaesthesia: a meta-analysis of individual patient data. Lancet Respir Med 4:272–280
Calfee CS, Delucchi K, Parsons PE et al (2014) Subphenotypes in acute respiratory distress syndrome: latent class analysis of data from two randomised controlled trials. Lancet Respir Med 2:611–620
Famous KR, Delucchi K, Ware LB et al (2017) Acute respiratory distress syndrome subphenotypes respond differently to randomized fluid management strategy. Am J Respir Crit Care Med 195:331–338
Shirasawa M, Fujiwara N, Hirabayashi S et al (2004) Receptor for advanced glycation end-products is a marker of type I lung alveolar cells. Genes Cells 9:165–174
Ware LB, Matthay MA (2000) The acute respiratory distress syndrome. N Engl J Med 342:1334–1349
Ware LB, Matthay MA (2001) Alveolar fluid clearance is impaired in the majority of patients with acute lung injury and the acute respiratory distress syndrome. Am J Respir Crit Care Med 163:1376–1383
Briot R, Frank JA, Uchida T et al (2009) Elevated levels of the receptor for advanced glycation end products, a marker of alveolar epithelial type I cell injury, predict impaired alveolar fluid clearance in isolated perfused human lungs. Chest 135:269–275
Calfee CS, Janz DR, Bernard GR et al (2015) Distinct molecular phenotypes of direct vs indirect ARDS in single-center and multicenter studies. Chest 147:1539–1548
Parsons PE, Eisner MD, Thompson BT et al (2005) Lower tidal volume ventilation and plasma cytokine markers of inflammation in patients with acute lung injury. Crit Care Med 33:1–6 (discussion 230–2)
Guo WA, Knight PR, Raghavendran K (2012) The receptor for advanced glycation end products and acute lung injury/acute respiratory distress syndrome. Intensive Care Med 38:1588–1598
Mukherjee TK, Mukhopadhyay S, Hoidal JR (2008) Implication of receptor for advanced glycation end product (RAGE) in pulmonary health and pathophysiology. Respir Physiol Neurobiol 162:210–215
Dreyfuss D, Soler P, Basset G, Saumon G (1988) High inflation pressure pulmonary edema. Respective effects of high airway pressure, high tidal volume, and positive end-expiratory pressure. Am Rev Respir Dis 137:1159–1164
Tschumperlin DJ, Oswari J, Margulies AS (2000) Deformation-induced injury of alveolar epithelial cells. Effect of frequency, duration, and amplitude. Am J Respir Crit Care Med 162:357–362
Nuckton TJ, Alonso JA, Kallet RH et al (2002) Pulmonary dead-space fraction as a risk factor for death in the acute respiratory distress syndrome. N Engl J Med 346:1281–1286
Funding
This work was supported by grants from the Auvergne Regional Council (“Programme Nouveau Chercheur de la Région Auvergne” 2013) (J.M. Constantin), the French Agence Nationale de la Recherche and Direction Générale de l’Offre de Soins (“Programme de Recherche Translationnelle en Santé” ANR-13-PRTS-0010) (M. Jabaudon), and by NHLBI Grants HL133390 and HL140026 (C.S. Calfee) and HL51856 (M.A. Matthay). The funders had no influence on the study design, conduct, and analysis or the preparation of this article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Jabaudon, M., Blondonnet, R., Pereira, B. et al. Plasma sRAGE is independently associated with increased mortality in ARDS: a meta-analysis of individual patient data. Intensive Care Med 44, 1388–1399 (2018). https://doi.org/10.1007/s00134-018-5327-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-018-5327-1