Abstract
Purpose
Anti-virulence strategies have not been evaluated for the prevention of bacterial infections. Prolonged colonization of intubated patients with Pseudomonas aeruginosa isolates producing high-levels of the quorum sensing (QS)-regulated virulence factor rhamnolipids has been associated with ventilator-associated pneumonia (VAP). In this pathogen, azithromycin reduces QS-regulated virulence. We aimed to assess whether azithromycin could prevent VAP in patients colonized by rhamnolipids producing isolates.
Methods
In a randomized, double-blind, multicenter trial, intubated colonized patients received either 300 mg/day azithromycin or placebo. Primary endpoint was the occurrence of P. aeruginosa VAP. We further identified those patients persistently colonized by isolates producing high-levels of rhamnolipids and therefore at the highest risk to develop VAP linked to this QS-dependent virulence factor.
Results
Ninety-two patients were enrolled; 43 azithromycin-treated and 42 placebo patients were eligible for the per-protocol analysis. In the per-protocol population, the occurrence of P. aeruginosa VAP was reduced in the azithromycin group but without reaching statistical significance (4.7 vs. 14.3 % VAP, p = 0.156). QS-dependent virulence of colonizing isolates was similarly low in both study groups, and only five patients in each arm were persistently colonized by high-level rhamnolipids producing isolates. In this high-risk subgroup, the incidence of VAP was reduced fivefold in azithromycin versus placebo patients (1/5 vs. 5/5 VAP, p = 0.048).
Conclusions
There was a trend towards reduced incidence of VAP in colonized azithromycin-treated patients. In addition, azithromycin significantly prevented VAP in those patients at high risk of rhamnolipid-dependent VAP, suggesting that virulence inhibition is a promising anti-microbial strategy.
Similar content being viewed by others
Introduction
Multi-drug-resistant bacteria represent a major medical threat, contributing to the deaths of increasing numbers of patients worldwide. To face this serious challenge, alternative strategies aiming at the inhibition of bacterial virulence have been proposed [1]. Due to the absence of selective pressure, such strategies are believed to minimize the risk of emergence of resistant clones [2, 3]. To our knowledge, however, no clinical study has demonstrated the feasibility of such an approach.
Pseudomonas aeruginosa is a leading cause of ventilator-associated pneumonia (VAP) [4–6]. This pathogen frequently develops resistance to available antimicrobial agents [7–9]. Improved treatments and/or preventive measures are urgently required since attributable mortality of this condition remains high [6]. Whereas almost all cases of P. aeruginosa-associated VAP are preceded by colonization of the respiratory tract, only 10–20 % of colonized patients eventually evolve to VAP [4]. Quorum sensing (QS) is a complex signaling network that allows coordinated gene expression according to cell density [1] and regulates many major virulence factors in P. aeruginosa [10]. As a consequence, QS is an ideal target for anti-virulence strategies. Macrolides, like azithromycin, are neither bactericidal nor bacteriostatic for P. aeruginosa at clinically achievable concentrations and are therefore unlikely to select resistant clones. However, at these concentrations, azithromycin inhibits QS in vitro [11–14]. Furthermore, we have shown that azithromycin reduces the expression of QS-circuit and target genes in patient [15]. Azithromycin has a favorable profile in terms of pharmacokinetics and of metabolism. Lung tissue distribution is high, enabling to reach QS-inhibitory concentrations (2–4 μg/ml) in bronchial mucosa, epithelial lining fluid, and sputum after a single 500-mg dose [16]. No clinical trial has previously investigated a virulence inhibition strategy for the prevention of bacterial infections. Thus, we decided to conduct a proof-of-concept study, designed as a multicenter, randomized, double-blind, placebo-controlled trial to evaluate azithromycin for the prevention of P. aeruginosa VAP in colonized mechanically ventilated patients.
Methods
Study population
This pilot, randomized, placebo-controlled, double-blind study (ANB 006#2001, ClinicalTrials.gov ID#NCT00610623) was designed to assess the efficacy of azithromycin as a quorum-sensing inhibitor in preventing the occurrence of P. aeruginosa pneumonia in ventilated patients with documented colonization in bronchial aspirates at study entry. We obtained approval for this multicenter European study by the respective local ethics committees and national agencies. Written consent from all patients or their legal representatives was obtained according to legal and ethical considerations. The study took place between November 2002 and November 2005. Twenty-one European centers participated in this trial; eight in France, four in Spain, two in Belgium, three in Poland, two in Serbia, and two in Switzerland. We screened mechanically ventilated patients for respiratory tract colonization by P. aeruginosa on alternate days. Eligible patients were adults between 18 and 75 years of age, hospitalized in the ICU under mechanical ventilation expected to be required for at least 3 days, with a reasonable surviving chance (Apache score between 10 and 25), and proven to be colonized by P. aeruginosa. Neutropenic patients and patients treated with immunosuppressive drugs were not eligible. Patients with ongoing P. aeruginosa infection, having received macrolides or antibiotics active against the colonizing P. aeruginosa isolate during the last 14 days were excluded. Patients with proven colonization by P. aeruginosa were randomized (D-1) and received either placebo or 300 mg per day iv azithromycin in a double-blind fashion for a maximum of 20 days (D1 to Dx). The 300-mg dose was selected taking into account a bioavailability of 37 % of the usual 500-mg oral dose, the need for reaching steady state at lung level within 3 days, and safety considerations.
During the study, the administration of antibiotics proven to be inactive against the isolated P. aeruginosa strains was allowed without restrictions. The administration of antibiotics with intrinsic activity against P. aeruginosa strains was only allowed if considered as mandatory. The prophylaxis study period was restricted to a maximum of 20 days, and reasons for early discontinuation included extubation, death, serious adverse events, or suspected P. aeruginosa VAP. The diagnosis of P. aeruginosa VAP was based on the clinical picture, X-ray scan, a pulmonary infection score (CPIS) ≥6, as well as a quantitative culture of a bronchoalveolar lavage fluid (BAL) yielding >104 CFU/ml P. aeruginosa, in the absence of other pathogenic bacterial species [17, 18]. An independent panel of three experts blinded to treatment confirmed the P. aeruginosa VAP cases.
Clinical sample collection
Starting the first day of proven colonization (D-1), daily tracheal aspirates (usually 0.3–5 ml) and P. aeruginosa strains were collected during the entire study period. Samples were frozen at −80°C on site within 15 min, and sent on dry ice to the reference research laboratory at the University Hospital Geneva, where all analyses were performed in a blind fashion.
Study design and statistical analysis
The study was initiated and financially supported by Anbics Corporation and a grant from the Swiss Ministry of Technology (Bundesamt für Berufsausbildung und Technologie, Kommission für Technologie und Innovation, KTI). The study objective as defined per protocol was to include ten P. aeruginosa VAP cases confirmed by the independent expert panel. This was based on the assumption of a 10 % occurrence of pneumonia in patients colonized by P. aeruginosa in the placebo group, with a total patient number between 100 and 200 assessable patients. The statistical objective defined in the study protocol (10 % occurrence of VAP in placebo group, two-sided, p = 0.10; Fisher’s exact test) required one P. aeruginosa VAP on azithromycin versus nine on placebo to reach significance (p = 0.016), or two P. aeruginosa VAP on azithromycin versus eight on placebo for a trend (p = 0.092). Because Anbics Corporation was dissolved prematurely, patient recruitment had to be stopped before reaching ten confirmed VAP cases. The investigators continued the laboratory and statistical analysis of all clinical samples, supported by grants from the Swiss National Science Foundation. The analysis was performed on the per-protocol basis. Using the placebo group of the present trial, we have described patients persistently colonized with P. aeruginosa strains producing elevated levels of the QS-dependent virulence factor rhamnolipids as a particular risk group to develop QS-related VAP [19, 20]. Therefore, we performed a second analysis in this sub-population. Two-sided Fisher’s exact tests were used to test differences between group rates. Odds ratios and 95 % confidence intervals were also calculated for all parameters. Two-sided independent-sample t tests were used to test differences between group-specific means, assuming equal variances.
Results
Study group description
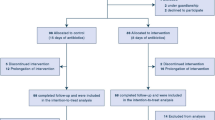
Out of 92 randomized patients, seven had to be excluded for major protocol violations including absence of P. aeruginosa colonization at study entry, treatment with macrolides or agents active against the colonizing P. aeruginosa isolates, and premature death (Fig. 1). Forty-two placebo and 43 azithromycin patients were included in the per-protocol analysis. The treatment groups were well balanced for race, age, gender, and severity-of-disease (measured by Apache II score) (Table 1). The most frequent medical reasons for requiring mechanical ventilation were surgical complications, deterioration of underlying diseases (congestive heart and/or acute respiratory failure) and acute respiratory distress syndrome, which were all equally distributed in both study groups. Twenty-three azithromycin and 28 placebo patients received a systemic antibiotherapy during the study period (p = 0.270, Table 2). Antibiotics devoid of activity against P. aeruginosa were administered to 21 azithromycin and 25 placebo patients (p = 0.386). Agents active against P. aeruginosa (ciprofloxacin, aminoglycosides, piperacillin/tazobactam, ceftazidime, aztreonam or carbapenems) were administered to seven azithromycin and eight placebo patients (p = 0.783, Table 2) for non-respiratory tract infections, which occurred mainly during the last days of the study. In six of these patients (three in each group), this treatment was active against the colonizing P. aeruginosa isolates. None of them developed P. aeruginosa VAP and only one placebo patient belonged to the high-risk group for developing QS-related VAP (as defined by persistent colonization with isolates producing high levels of the QS-dependent virulence factor rhamnolipids, see below). The colonizing isolates of the nine other patients (four azithromycin and five placebo) were found to be resistant to the antibiotic used. Azithromycin exposure did not lead to an MIC increase comparing the initial and last P. aeruginosa isolates (data not shown). As compared to the placebo group, no increased incidence of infections, whether due to P. aeruginosa or other bacteria, was detected at other sites in the azithromycin-treated group. We observed no significant differences in the distribution of P. aeruginosa genotypes or virulence determinants (production of the QS-dependent virulence factors elastase and rhamnolipids, presence of the Pseudomonas pathogenicity islands PAI-1 and PAI-2, and the phospholipase gene ExoU) between the two study groups (Table 3, for details see online supplementary data). We have recently shown that patients can be classified into three risk-categories for VAP according to the production of rhamnolipids of their colonizing isolates [20]. Whereas 11 placebo [20] and seven azithromycin-treated (Supplementary Fig. 1B and C) patients were persistently colonized by isolates producing low levels, 13 placebo and 18 treated patients were colonized by isolates producing intermediate levels, and five patients in each study group were persistently colonized by isolates producing high levels of rhamnolipids (Table 3).
Primary clinical endpoints
In the intention-to-treat analysis, the mean duration of treatment was 9.5 days for the azithromycin-treated and 10.8 days for the placebo groups (Table 4). Eight azithromycin and ten placebo patients were treated for the entire study period of 20 days (p = 0.604). Reasons for early discontinuation were similar between the treatment groups when assessing for serious adverse events, non-pseudomonal pneumonia, extubation, and death unrelated to P. aeruginosa VAP (Table 4). Concerning the primary study end-point on the per-protocol population, P. aeruginosa VAP occurred in 2/43 azithromycin-treated and in 6/42 placebo patients (4.7 %, respectively, 14.3 %, odds ratio; 3.42, CI 95 %; 0.65–18.00, p = 0.156) during the prophylaxis study window (Table 5). One patient of each study group (placebo patient #13116 [20] and treated patient #13115, supplementary Fig. 1A) colonized by ExoU-positive isolates developed P. aeruginosa VAP.
Clinical efficacy of azithromycin in patients colonized by isolates producing the QS-dependent virulence factor rhamnolipids
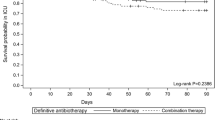
It became apparent after study completion that many patients of both study arms were colonized by either completely or partially QS-deficient isolates. Therefore, many patients were at low risk of developing “QS-dependent” VAP. QS-inhibition by azithromycin was likely to be ineffective, respectively of less clinical benefit to these patients, as compared to patients colonized by fully QS-proficient isolates [19, 20]. We therefore performed a subanalysis including only those patients in whom the QS inhibition by azithromycin could be effective. First, we compared the occurrence of VAP in patients colonized by isolates producing intermediate or high levels of rhamnolipids (Table 3). In this subgroup, P. aeruginosa VAP occurred in 5/18 placebo and in 1/23 azithromycin-treated patients (Table 5) (odds ratio 8.46, CI 95 % 0.89–80.59, p = 0.07). Using the placebo group of the present study, we have previously shown that patients at highest risk for developing QS-related VAP are those persistently colonized by isolates producing high levels of rhamnolipids [15]. Considering only this high-risk subpopulation, P. aeruginosa VAP occurred in 1/5 azithromycin-treated and in 5/5 placebo patients (20 vs. 100 %, p = 0.047) during the prophylaxis study window (Table 5). One azithromycin-treated patient developed acute P. aeruginosa pneumonia outside of the prophylaxis study window (48 h after his extubation and study drug discontinuation).
Safety and tolerance
The safety profile was assessed in 92 patients up to 7 days after completion of the prophylaxis study window. The adverse event (AE) profile was balanced between the azithromycin and placebo groups. At least one treatment-related AE was reported for 11 versus 13 % of patients and serious treatment-related adverse events occurred in one azithromycin (bradycardia and bronchospasm) and two placebo patients (ventricular fibrillation, massive pneumonic infiltrate). In the intention-to-treat set, nine azithromycin and six placebo patients died, while in the per-protocol set, six patients of each treatment group died. None of these deaths was considered drug-related, and the majority (9/12) occurred after cessation of treatment (Table 4). No increased risk for infections and/or emergence of resistance to antibiotics was identified. A single case of pseudomembranous colitis occurred in an azithromycin-treated patient.
Discussion
This multicenter, placebo-controlled trial of 92 intubated patients colonized by P. aeruginosa is the first pilot trial investigating the efficacy of an anti-virulence strategy to prevent a major infection. Such new interventions are desperately needed in view of the rising concern about infections caused by multi-resistant bacteria. Unfortunately, the study population was smaller than initially planned as a result of a premature study stop due to discontinuation of financial support. Consequently, the study was not sufficiently powered to detect statistically significant differences in the incidence of VAP in the per-protocol analysis.
We used the macrolide azithromycin, known to be neither bactericidal nor bacteriostatic on P. aeruginosa, but which inhibits QS-dependent virulence [11, 14]. We applied special care to determine both QS-dependent and QS-independent virulence determinants of sequential isolates of both study populations since any differences could have potentially influenced the efficacy of an anti-virulence intervention. Both study populations were colonized by isolates with similar virulence profiles, however, to our surprise, many patients were colonized by QS-deficient isolates. Studies performed on the placebo group of the present trial have shown that these patients are at low risk of developing QS-related VAP [19, 20]. Obviously such patients could not be anticipated to benefit from the anti-QS activity of azithromycin, reducing further the population under survey. Added to the premature stop of patient recruitment, this might explain the absence of a more significant advantage in the per-protocol analysis, although an encouraging difference was seen (4.7 vs. 14.3 %). Moreover the complex work performed on the tracheal isolates and aspirates from the placebo group allowed us to dissect the role of QS phenotypes after completion of the study. Detailed discussions concerning the implications of genotypes and virulence phenotypes, as well as dynamics of P. aeruginosa populations, have been published elsewhere [15, 19–21]. It became apparent that only patients persistently colonized by isolates producing elevated levels of the QS-dependent virulence factors rhamnolipids were actually at high risk of developing VAP [20]. While we observed a trend (p = 0.07) towards less VAP cases in the subgroup of azithromycin-treated patients colonized by isolates producing detectable levels of rhamnolipids, a protective effect of azithromycin became apparent (p = 0.048) in those treated patients who were colonized by high rhamnolipid producers, potentially preventing four out of five of these high-risk patients from developing VAP.
Two patients, one in the placebo (#13116) and one in the azithromycin-treated group (#13115), developed VAP while colonized by lasR-rhlR double mutants. These isolates carried the exoU gene encoding a phospholipase, which is translocated through the type III secretion system (TTSS) and associated with increased mortality from pneumonia [22]. However, whether colonization with exoU isolates is a risk factor for progression from colonization to VAP is unknown, and our results suggest that this is not the case [20]. It appears that exoU is not associated with the risk for VAP but with its severity. Interestingly, the TTSS is negatively controlled by QS [23, 24]. Therefore, inhibition of QS by azithromycin should have potentially increased the expression of type III cytotoxins, and therefore the risk for severe VAP. In the present study, 11 placebo and nine azithromycin-treated patients were colonized by strains harboring exoU. As only one patient of each study group developed VAP while colonized by exoU-positive isolates, this cannot have accounted for the increased incidence of VAP in the placebo group.
We have previously shown that a natural cooperative selection process during P. aeruginosa colonization led to progressive replacement of QS-proficient by QS-deficient isolates (cheater cells) due to increase in frequency of lasR mutants in the placebo group [19]. Remarkably, using the patients of the present study, we showed that QS inhibition by azithromycin reduced this natural selection towards QS-deficient isolates [15]. As a consequence, treated patients were more frequently colonized at study end with QS-competent, highly virulent bacteria [15]. This potentially increased their risk of infection once the prophylaxis was stopped, as suggested by the occurrence of P. aeruginosa pneumonia outside of the prophylaxis study window in one treated patient. It should induce caution upon use of antivirulence strategies in general, and special care should be taken in future studies in covering for the risk of post-ventilation infection by extending prophylaxis after extubation, at least for a few days.
Our study has several limitations. (1) The study was underpowered to detect differences in the per-protocol analysis and caution has to be taken when extrapolations are made from analyses performed on sub-groups defined after study closure. (2) As we did not measure rhamnolipid concentrations in respiratory secretions, we cannot fully exclude whether part of the beneficial effect of azithromycin could have been mediated through its immunomodulatory activities, or through modifications of the lung-resident microbial flora and not through inhibition of rhamnolipid production [25, 26]. However, one would expect that any immunosuppressant activity of azithromycin would have potentially worsened the progression to and/or severity of an acute infection such as P. aeruginosa VAP. This was not observed in our trial. Moreover, the resident microbial flora is more likely to protect from P. aeruginosa VAP, and therefore its elimination by azithromycin should have increased the risk of VAP instead of protecting from it. Furthermore, using the patients of the present trial, we have previously demonstrated that QS-gene expression, required for rhamnolipid production, was reduced in the azithromycin-treated as compared to the placebo group [15]. These results provide indirect proof that the prevention of VAP in patients colonized by QS-proficient isolates most likely resulted from inhibition of QS-gene expression, and reduction of rhamnolipid production, by azithromycin. (3) Even if the administration of antibiotics was strictly controlled during the entire study, three patients in each group received antibiotics active against their colonizing isolates. Only one of these patients was colonized by high-rhamnolipid producers and received placebo. Therefore, it is unlikely that this might have influenced the outcome of the subgroup analysis in favor of the azithromycin group. (4) We did not investigate whether azithromycin potentially modifies the antibiotic susceptibly of the gut flora. However, azithromycin did not select for increased resistance among P. aeruginosa isolates and did not elicit an increased incidence of infections with resistant non-Pseudomonal strains.
In conclusion, this study is the first randomized placebo-controlled trial evaluating the efficacy of an anti-virulence intervention for the prevention of a major nosocomial infection. Our results suggest that azithromycin might not prevent P. aeruginosa VAP in all intubated colonized patients. However, a subgroup of patients, at high risk of developing P. aeruginosa VAP as a consequence of their colonization by rhamnolipid-producing isolates, might benefit from the QS-blocking activity of azithromycin. These data stress the need for a large phase IIb clinical trial restricted to patients colonized by QS-proficient isolates to further establish safety and efficacy of QS inhibition by azithromycin to prevent P. aeruginosa VAP.
References
Van Delden C, Iglewski BH (1998) Cell-to-cell signaling and Pseudomonas aeruginosa infections. Emerg Infect Dis 4:551
Smith RS, Iglewski BH (2003) Pseudomonas aeruginosa quorum sensing as a potential antimicrobial target. J Clin Invest 112:1460–1465
Rasmussen TB, Givskov M (2006) Quorum sensing inhibitors: a bargain of effects. Microbiology 152:895–904
Chastre J, Fagon JY (2002) Ventilator-associated pneumonia. Am J Respir Crit Care Med 165:867
Torres A, Ewig S, Lode H, Carlet J (2009) Defining, treating and preventing hospital acquired pneumonia: European perspective. Intensive Care Med 35:9–29
Nguile-Makao M, Zahar JR, Francais A, Tabah A, Garrouste-Orgeas M, Allaouchiche B, Goldgran-Toledano D, Azoulay E, Adrie C, Jamali S, Clec’h C, Souweine B, Timsit JF (2010) Attributable mortality of ventilator-associated pneumonia: respective impact of main characteristics at ICU admission and VAP onset using conditional logistic regression and multi-state models. Intensive Care Med 36:781–789
Hancock RE, Speert DP (2000) Antibiotic resistance in Pseudomonas aeruginosa: mechanisms and impact on treatment. Drug Resist Updat 3:247–255
Mesaros N, Nordmann P, Plesiat P, Roussel-Delvallez M, Van Eldere J, Glupczynski Y, Van Laethem Y, Jacobs F, Lebecque P, Malfroot A, Tulkens PM, Van Bambeke F (2007) Pseudomonas aeruginosa: resistance and therapeutic options at the turn of the new millennium. Clin Microbiol Infect Off Publ Eur Soc Clin Microbiol Infect Dis 13:560–578
van Delden C (2007) Pseudomonas aeruginosa bloodstream infections: how should we treat them? Int J Antimicrob Agents 30(Suppl 1):S71–S75
van Delden C (2004) Virulence factors in Pseudomonas aeruginosa. In: Ramos JL (ed) The Pseudomonads. Kluwer Academic, New York, pp 3–46
Tateda K, Comte R, Pechère JC, Köhler T, Yamaguchi K, Van Delden C (2001) Azithromycin inhibits quorum sensing in Pseudomonas aeruginosa. Antimicrob Agents Chemother 45:1930–1933
Favre-Bonté S, Köhler T, Van Delden C (2003) Biofilm formation by Pseudomonas aeruginosa: role of the C4-HSL cell-to-cell signal and inhibition by azithromycin. J Antimicrob Chemother 52:598–604
Nalca Y, Jansch L, Bredenbruch F, Geffers R, Buer J, Haussler S (2006) Quorum-sensing antagonistic activities of azithromycin in Pseudomonas aeruginosa PAO1: a global approach. Antimicrob Agents Chemother 50:1680–1688
Hoffmann N, Lee B, Hentzer M, Rasmussen TB, Song Z, Johansen HK, Givskov M, Hoiby N (2007) Azithromycin blocks quorum sensing and alginate polymer formation and increases the sensitivity to serum and stationary-growth-phase killing of Pseudomonas aeruginosa and attenuates chronic P. aeruginosa lung infection in Cftr(-/-) mice. Antimicrob Agents Chemother 51:3677–3687
Kohler T, Perron GG, Buckling A, van Delden C (2010) Quorum sensing inhibition selects for virulence and cooperation in Pseudomonas aeruginosa. PLoS Pathog 6:e1000883
Baldwin DR, Wise R, Andrews JM, Ashby JP, Honeybourne D (1990) Azithromycin concentrations at the sites of pulmonary infection. Eur Respir J Off J Eur Soc Clin Respir Physiol 3:886–890
Pugin J, Auckenthaler R, Mili N, Janssens JP, Lew PD, Suter PM (1991) Diagnosis of ventilator-associated pneumonia by bacteriologic analysis of bronchoscopic and nonbronchoscopic “blind” bronchoalveolar lavage fluid. Am Rev Respir Dis 143:1121
Singh N, Rogers P, Atwood CW, Wagener MM, Yu VL (2000) Short-course empiric antibiotic therapy for patients with pulmonary infiltrates in the intensive care unit. A proposed solution for indiscriminate antibiotic prescription. Am J Respir Crit Care Med 162:505–511
Kohler T, Buckling A, van Delden C (2009) Cooperation and virulence of clinical Pseudomonas aeruginosa populations. Proc Natl Acad Sci USA 106:6339–6344
Kohler T, Guanella R, Carlet J, van Delden C (2010) Quorum sensing-dependent virulence during Pseudomonas aeruginosa colonisation and pneumonia in mechanically ventilated patients. Thorax 65:703–710
Kohler T, Donner V, van Delden C (2010) Lipopolysaccharide as shield and receptor for R-pyocin-mediated killing in Pseudomonas aeruginosa. J Bacteriol 192:1921–1928
Hauser AR, Cobb E, Bodi M, Mariscal D, Valles J, Engel JN, Rello J (2002) Type III protein secretion is associated with poor clinical outcomes in patients with ventilator-associated pneumonia caused by Pseudomonas aeruginosa. Crit Care Med 30:521
Singh G, Wu B, Baek MS, Camargo A, Nguyen A, Slusher NA, Srinivasan R, Wiener-Kronish JP, Lynch SV (2010) Secretion of Pseudomonas aeruginosa type III cytotoxins is dependent on pseudomonas quinolone signal concentration. Microb Pathog 49:196–203
Bleves S, Soscia C, Nogueira-Orlandi P, Lazdunski A, Filloux A (2005) Quorum sensing negatively controls type III secretion regulon expression in Pseudomonas aeruginosa PAO1. J Bacteriol 187:3898–3902
Tamaoki J, Kadota J, Takizawa H (2004) Clinical implications of the immunomodulatory effects of macrolides. Am J Med 117 Suppl 9A:5S–11S
Amsden GW (2005) Anti-inflammatory effects of macrolides–an underappreciated benefit in the treatment of community-acquired respiratory tract infections and chronic inflammatory pulmonary conditions? J Antimicrob Chemother 55:10–21
Acknowledgments
We express our gratitude to the responsible physicians in the participating centers as well as to the nurses and medical staff involved in this multicenter study. The following 21 centers and investigators contributed to the trial: in France, Hôpital Dupuytren (Limoges, Dr. B. François), Hôpital St. Joseph (Paris, Dr. B. Misset, Dr. J. Carlet), Hôpital Cochin (Paris, Dr. A. Cariou), Centre Hospitalier Montauban (Montauban, Dr. J. Roustan), Hôpital Calmette (Lille, Dr. A. Durocher), Hôpital Jean Minjoz (Besançon, Dr. G. Capellier), Hôpital Bichat (Paris, Dr. C. Paugam, Dr. J.-F. Timsit); in Spain: Hospital del Mar (Barcelona, Dr. F. Alvarez-Lerma), Joan XXIII University Hospital (Tarragona, Dr. J. Rello), Hospital Vall d’Hebron (Barcelona, Dr M. Palomar), Hospital Universitario San Dureta (Palma de Mallorca, Dr. J.I. Ayesteran); in Serbia: Hospital Belgrade (Belgrade, Dr. M. Gvozdenovic), Clinical Center of Serbia (Belgrade, Dr. B. Milakovic); in Belgium: Hôpital Universitaire Liège (Liège, Dr. P. Damas), Hôpital St. Pierre (Ottignies, Dr. T. Dugernier); in Poland: Wojewodzki Hospital (Sosnowiec, Dr. L. Krawczyk), Warsaw Medical University (Warsaw, Dr. A. Kański), Wojewodzki Hospital (Krakow, Dr. T. Zelazni); in Switzerland: Centre Hospitalier Universitaire Vaudois (Lausanne, Dr. R. Choléro, Dr. A.M. Schaller), Centre Hospitalier Universitaire de Genève (Genève, Dr. R. Garbino). This work was supported by a clinical grant from Anbics Corporation, by a grant from the Swiss Ministry of Technology, and by the Swiss National Science Foundation (grants 4049-063239 to TK and CVD, grant 320000-108106 to CVD).
Conflicts of interest
The authors declare that they have no competing financial interests with this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is discussed in the editorial available at: doi:10.1007/s00134-012-2561-9.
J.-C. Pechère: Deceased 29th November 2008.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
van Delden, C., Köhler, T., Brunner-Ferber, F. et al. Azithromycin to prevent Pseudomonas aeruginosa ventilator-associated pneumonia by inhibition of quorum sensing: a randomized controlled trial. Intensive Care Med 38, 1118–1125 (2012). https://doi.org/10.1007/s00134-012-2559-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-012-2559-3