Abstract
Objective
To compare the efficiency of an Aeroneb Pro vibrating plate and an Atomisor MegaHertz ultrasonic nebulizer for providing ceftazidime distal lung deposition.
Design
In vitro experiments. One gram of cetazidime was nebulized in respiratory circuits and mass median aerodynamic diameter of particles generated by ultrasonic and vibrating plate nebulizers was compared using a laser velocimeter. In vivo experiments. Lung tissue concentrations and extrapulmonary depositions were measured in ten anesthetized ventilated piglets with healthy lungs that received 1 g of ceftazidime by nebulization with either an ultrasonic (n = 5), or a vibrating plate (n = 5) nebulizer.
Setting
A two-bed Experimental Intensive Care Unit of a University School of Medicine.
Intervention
Following sacrifice, 5 subpleural specimens were sampled in dependent and nondependent lung regions for measuring ceftazidime lung tissue concentrations by high-performance liquid chromatography.
Measurements and results
Mass median aerodynamic diameters generated by both nebulizers were similar with more than 95% of the particles between 0.5 and 5 μm. Lung tissue concentrations were 553 ± 123 [95% confidence interval: 514–638] μg g−1 using ultrasonic nebulizer, and 452 ± 172 [95% confidence interval: 376–528] μg g−1 using vibrating plate nebulizers (NS). Extrapulmonary depositions were, respectively, of 38 ± 5% (ultrasonic) and 34 ± 4% (vibrating plate) (NS).
Conclusions
Vibrating plate nebulizer is comparable to ultrasonic nebulizers for ceftazidime nebulization. It may represent a new attractive technology for inhaled antibiotic therapy.
Similar content being viewed by others
Introduction
Optimisation of antibiotic nebulization during mechanical ventilation requires reduced tidal volume, low respiratory frequency, prolonged inspiration and an aerodynamic diameter of aerosolized particles ranging between 1 and 5 μm [1–3]. Jet nebulizers are considered as the reference for delivering bronchodilatators and antibiotics to the tracheobronchial tree of patients with asthma and cystic fibrosis [2]. Two comparative studies, however, suggest that jet nebulizers are less efficient than ultrasonic or vibrating plate nebulizers as far as distal lung deposition is concerned. In mechanically ventilated patients receiving aerosolized radiolabelled albumin, a significant higher lung deposition was obtained with a DP100 ultrasonic nebulizer compared to a Medic-Aid jet nebulizer Medic-Aid [4]. In ventilated neonate animals receiving radiolabelled aerosols, a higher lung deposition was obtained with an Aeroneb Professional vibrating plate nebulizer compared to a MistyNeb jet nebulizer [5]. High lung tissue concentrations of amikacin and ceftazidime have been reported following ultrasonic nebulization in experimental and human ventilator-associated pneumonia [6–8]. Ultrasonic nebulizers, however, are voluminous and quartz vibrations increase the temperature into the nebulizer chamber, a factor that may alter chemical properties of antibiotics. Vibrating plate nebulizers belong to the last generation of nebulizers. The aerosol is generated from a vibrating plate with multiple apertures, whose diameter determines aerodynamic diameter of aerosolized particles [5]. Because of their small size, vibrating plate nebulizers are quite ergonomic and are easy to use.
The aim of this experimental study was to compare the efficiency of an Aeroneb Pro vibrating plate and an Atomisor MegaHertz ultrasonic nebulizer for aerosolizing ceftazidime to the distal lung of healthy mechanically ventilated piglets.
Materials and methods
In vitro measurement of mass median aerodynamic diameter of particles (MMAD)
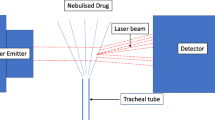
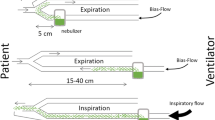
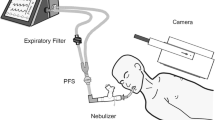
The experimental set-up including the ventilator, the ventilator settings used throughout the in vitro and in vivo experiments, the respiratory circuits and the nebulizers is shown in Fig. 1. One gram of ceftazidime was nebulized continuously into the circuits by the ultrasonic nebulizer (Atomisor MegaHertz®; Diffusion Technique Française, Saint-Etienne, France) and by the vibrating plate nebulizer (Aeroneb Pro®; Aerogen Nektar Corporation, Galway, Ireland). Without connecting the animals to the ventilator and according to a technique previously described [9] MMAD was measured at the outlet of the nebulizer and at the distal tip of the endotracheal tube using a laser velocimeter (Malvern Instrument, Worcestershire, UK).
Experimental design for ceftazidime nebulization. Respiratory circuits were composed of a Cesar ventilator (Taema, Antony, France), two 150-cm-long inspiratory and expiratory circuits, a Y piece and one 15- or 40-cm long connecting tube. One gram of ceftazidime was nebulized continuously in the circuits by the ultrasonic nebulizer, positioned 40 cm from the Y piece and by the vibrating plate nebulizer positioned 15 cm from the Y piece. The connecting tube between nebulizer and Y piece serves as a reservoir containing the aerosol generated during the expiratory phase, which is entrained into the tracheobronchial tree during the next inspiration (bolus effect). A filter with a pore size of 0.2 μm (Hygrobac; Mallinckrodt Medical, Mirandola, Italy) is placed on the expiratory limb to collect expired aerosolized particles. According to previous studies that identified ventilator settings promoting distal lung deposition of aerosolized particles [1–3], the following ventilator settings were used: absence of heat and moisture exchanger, volume controlled mode, administration of a constant and low inspiratory flow (9 l/min), a tidal volume of 300 ml, respiratory rate of 15 breaths/min, inspiratory/expiratory ratio of 50%, an end-inspiratory pause representing 20% of the duty cycle, positive end-expiratory pressure of 5 cmH2O and FiO2 0.21
Animal preparation
Ten healthy bred domestic Largewhite-Landrace piglets, aged 3 months and weighting 20 ± 1 kg, were anesthetized and orotracheally intubated with a 7.5 Hi-Lo Jet Mallinckrodt tube (Mallinckrodt Incorporation, Argyle, NY) and ventilated as described in Fig. 1. The study was conducted according to French law concerning experimental studies.
Aerosol generation
All piglets received 1 g of ceftazidime powder diluted in sterile water. In five animals, nebulization was performed by the ultrasonic nebulizer and in five by the vibrating plate nebulizer. As shown in Fig. 1, experiments were performed without humidification of inspired gas [10] using ventilator settings recommended for promoting lung deposition. In addition, a 65%/35% helium–oxygen mixture was used for ventilation during the period of nebulization to optimize lung deposition as previously demonstrated [8].
Assessment of ceftazidime lung tissue concentrations
Piglets were killed by exsanguination 15 min after completion of nebulization as previously described [8]. The lungs were then removed from the thorax and five subpleural lung specimens were excised from the upper lobe, the middle lobe, the apical-dependent segment of the lower lobe, the anterior-nondependent segment of lower lobe, and the postero-caudal segment of lower lobe. Tissue samples were cryomixed in liquid nitrogen, weighed and homogenized, and ceftazidime concentrations were measured in a blinded fashion by high-performance liquid chromatography (HPLC) with correction for contaminating blood [8].
Assessment of extrapulmonary deposition
At the end of the experiment, nebulizer chamber, inspiratory and expiratory circuits, Y piece, connecting tube, endotracheal tube, and expiratory filter were rinsed, respectively, with 1 l of distilled water. The amount of ceftazidime of the different parts was separately measured by HPLC. Extrapulmonary deposition was defined as the total amount of ceftazidime recovered from respiratory circuits. Percentage of total extrapulmonary deposition was calculated as the amount of ceftazidime recovered in the different respiratory circuits divided by the dose of ceftazidime inserted in the nebulizer chamber. Percentage of ceftazidime entering the respiratory system was defined as 100 minus percentage of extrapulmonary deposition.
Statistical analysis
The present study was considered as a pilot study and power calculation was not performed. The 95% confidence interval of the lung tissue concentrations was calculated as recommended [11]. Data were analysed using Sigmastat Software (SPSS, Inc., San Raphael, CA). The normal distribution of data was verified by a Kolmogorov–Smirnov test. Extrapulmonary depositions obtained after nebulization performed by ultrasonic and vibrating plate nebulizers were compared by a paired Student’s t test. Lung tissue ceftazidime concentrations measured in the two groups of piglets (ultrasonic and vibrating plate nebulizers) in different lung segments were compared by a two-way analysis of variance for repeated measures. A p value less than 0.05 was considered as significant. All data are expressed as mean ± SD.
Results
In vitro experiments
As shown in Table 1, MMAD was not influenced by nebulizer, tidal volume and site of measurement. As shown in Table 2, extrapulmonary deposition was similar between ultrasonic and vibrating plate nebulizers. Extrapulmonary deposition was predominant in the nebulizer chamber during ultrasonic nebulization and predominant in the inspiratory circuits during vibrating plate nebulization.
Lung tissue concentrations of ceftazidime
The lungs were pink and healthy. Ceftazidime lung tissue concentrations were homogeneously distributed between nondependent and dependent pulmonary segments, whatever the technique used (Fig. 2). Lung tissue concentrations were not statistically different among lung segments and between vibrating plate and ultrasonic nebulizers. Mean lung tissue concentrations were 452 ± 172 [95% confidence interval: 376–528] μg g−1 and 553 ± 123 [95% confidence interval: 514–638] μg g−1).
Regional distribution of ceftazidime lung tissue concentrations in dependent (B3, B6 and B10) and nondependent (B2 and B8) lung segments after nebulization of 1 g of ceftazidime by ultrasonic and vibrating plate nebulizers. Lung tissue concentrations are homogeneously distributed among the different lung segments. USN ultrasonic nebulizer, VPN vibrating plates nebulizer
Discussion
The present study demonstrates that ultrasonic and vibrating plate nebulizers have a comparable efficiency for nebulizing 1 g of ceftazidime in anesthetized and ventilated healthy piglets.
MMAD and extrapulmonary deposition resulting from ultrasonic and vibrating plate nebulizers
Aerosol particle size is one of the key factors influencing lung deposition. Particles bigger than 5 μm rapidly impact respiratory circuits and large airways. The optimal MMAD ranges between 0.5 and 5 μm [12] and it is well known that nebulizer technology influences MMAD [13, 14]. In the present study, ultrasonic and vibrating plate nebulizers were equivalent for producing a high percentage of particle size ranging between 0.5 and 5 μm.
Bench studies have shown that extrapulmonary deposition ranges between 60 and 80% with optimized ventilatory settings [14, 15]. In the present study, in vivo extrapulmonary deposition was 38 and 34% for ultrasonic and vibrating plate nebulizers, respectively. Discrepancies in aerosol deposition between in vitro and in vivo conditions have been previously reported [16]. During in vitro conditions, the filter serving to estimate “pulmonary” deposition captures small particles and increases respiratory resistance, both effects influencing extrapulmonary deposition [17]. Use of a helium–oxygen mixture in the present study was aimed at converting turbulent into laminar flows in order to further decrease extrapulmonary deposition and increase pulmonary deposition [8, 18].
Despite the reduction of the inspiratory circuit volume, deposition into the inspiratory circuit was higher with vibrating plate nebulizer, likely because local turbulences resulting from vibrating plate technology are greater than those generated by ultrasonic vibration and promote impaction of aerosolized particles into the inspiratory circuits [19]. Deposition into the chamber of the ultrasonic nebulizer was greater because of its larger volume.
Comparative ceftazidime lung tissue deposition
Lung tissue deposition of ceftazidime was similar between vibrating plate and ultrasonic nebulizer. A recent bench study has also shown comparable aerosol delivery between both techniques [14]. As discussed above, 60% of the dose inserted in the nebulizer chamber entered the respiratory system. Because nebulization was performed in healthy lungs, as previously demonstrated [12], a homogeneous distribution of ceftazidime was found (Fig. 2). Based on this homogeneous distribution, an approximate estimate of the total dose of ceftazidime reaching the distal lung can be calculated. The weight of two exsanguinated 20-kg piglets with healthy lungs, is around 200 g [20]. As mean lung tissue concentrations were measured at 553 and 452 μg/g with ultrasonic and vibrating plate nebulizers, it can be assumed that 553 μg/g × 200 g = 110.6 mg reached the distal lung with the ultrasonic nebulizer and 452 μg/g × 200 g = 90.4 mg with the vibrating plate nebulizer. Therefore, around 50% of the nebulized dose reaches proximal airways and 10% the lung parenchyma, a result higher than that previously reported [4].
In conclusion, in ventilated piglets with healthy lungs, vibrating plate nebulizers and ultrasonic nebulizers have a similar efficiency: among 60% of the ceftazidime dose delivered to the respiratory system, 50% reach the tracheobronchial tree and 10% the distal lung. Because of better ergonomics and simplicity of use, vibrating plate nebulizers may provide an attractive alternative for inhaled antibiotic therapy as a treatment of ventilator-associated pneumonia.
References
O’Riordan TG, Palmer LB, Smaldone GC (1994) Aerosol deposition in mechanically ventilated patients. Optimizing nebulizer delivery. Am J Respir Crit Care Med 149:214–219
Dhand R, Mercier E (2007) Effective inhaled drug administration to mechanically ventilated patients. Expert Opin Drug Deliv 4:47–61
Rouby JJ, Goldstein I, Lu Q (2006) Inhaled antibiotic therapy. In: Tobin MJ (ed) Principles and practice of mechanical ventilation. McGraw-Hill Medical Publishing Division, New York, pp 1311–1332 Chapter 64
Harvey CJ, O’Doherty MJ, Page CJ, Thomas SH, Nunan TO, Treacher DF (1997) Comparison of jet and ultrasonic nebulizer pulmonary aerosol deposition during mechanical ventilation. Eur Respir J 10:905–909
Dubus JC, Vecellio L, De Monte M, Fink JB, Grimbert D, Montharu J, Valat C, Behan N, Diot P (2005) Aerosol deposition in neonatal ventilation. Pediatr Res 58:10–14
Bressolle F, de la Coussaye JE, Ayoub R, Fabre D, Gomeni R, Saissi G, Eledjam JJ, Galtier M (1992) Endotracheal and aerosol administrations of ceftazidime in patients with nosocomial pneumonia: pharmacokinetics and absolute bioavailability. Antimicrob Agents Chemother 36:1404–1411
Goldstein I, Wallet F, Nicolas-Robin A, Ferrari F, Marquette CH, Rouby JJ (2002) Lung deposition and efficiency of nebulized amikacin during Escherichia coli pneumonia in ventilated piglets. Am J Respir Crit Care Med 166:1375–1381
Tonnellier M, Ferrari F, Goldstein I, Sartorius A, Marquette CH, Rouby JJ (2005) Intravenous versus nebulized ceftazidime in ventilated piglets with and without experimental bronchopneumonia: comparative effects of helium and nitrogen. Anesthesiology 102:995–1000
Elman M, Goldstein I, Marquette CH, Wallet F, Lenaour G, Rouby JJ (2002) Influence of lung aeration on pulmonary concentrations of nebulized and intravenous amikacin in ventilated piglets with severe bronchopneumonia. Anesthesiology 97:199–206
Miller DD, Amin MM, Palmer LB, Shah AR, Smaldone GC (2003) Aerosol delivery and modern mechanical ventilation: in vitro/in vivo evaluation. Am J Respir Crit Care Med 168:1205–1209
Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig LM, Moher D, Rennie D, de Vet HC, Lijmer JG (2003) The STARD statement for reporting studies of diagnostic accuracy: explanation and elaboration. Ann Intern Med 138:W1–W12
Goldstein I, Wallet F, Robert J, Becquemin MH, Marquette CH, Rouby JJ (2002) Lung tissue concentrations of nebulized amikacin during mechanical ventilation in piglets with healthy lungs. Am J Respir Crit Care Med 165:171–175
Faurisson F, Dessanges JF, Grimfeld A, Beaulieu R, Kitzis MD, Peytavin G, Lefevre JP, Farinotti R, Sautegeau A (1996) Comparative study of the performance and ergonomics of nebulizers in cystic fibrosis. Rev Mal Respir 13:155–162
Pedersen KM, Handlos VN, Heslet L, Kristensen HG (2006) Factors influencing the in vitro deposition of tobramycin aerosol: a comparison of an ultrasonic nebulizer and a high-frequency vibrating mesh nebulizer. J Aerosol Med 19:175–183
O’Riordan TG, Amram JC (1997) Effect of nebulizer configuration on delivery of aerosolized tobramycin. J Aerosol Med 10:13–23
Fink JB, Dhand R, Grychowski J, Fahey PJ, Tobin MJ (1999) Reconciling in vitro and in vivo measurements of aerosol delivery from a metered-dose inhaler during mechanical ventilation and defining efficiency-enhancing factors. Am J Respir Crit Care Med 159:63–68
Jandre FC, Carvalho AR, Pino AV, Giannella-Neto A (2005) Effects of filtering and delays on the estimates of a nonlinear respiratory mechanics model. Respir Physiol Neurobiol 148:309–314
Goode ML, Fink JB, Dhand R, Tobin MJ (2001) Improvement in aerosol delivery with helium–oxygen mixtures during mechanical ventilation. Am J Respir Crit Care Med 163:109–114
Dhand R (2002) Nebulizers that use a vibrating mesh or plate with multiple apertures to generate aerosol. Respir Care 47:1406–1416; discussion 1416–1408
Sartorius A, Lu Q, Vieira S, Tonnellier M, Lenaour G, Goldstein I, Rouby JJ (2007) Mechanical ventilation and lung infection in the genesis of air-space enlargement. Crit Care 11:R14
Acknowledgments
The authors thank Benoît Lecuelle, Arnold Dive and Michel Pottier, Département Hospitalo-Universitaire de Recherche Expérimentale and INSERM U 416 of Institut Pasteur, University of Lille, France for the preparation of the animals.
Author information
Authors and Affiliations
Corresponding author
Additional information
Vibrating plate nebulizers were provided by Aerogen Nektar Corporation, Galway, Ireland. Other support was provided from institutional and/or departmental source. None of the authors received any financial support from the manufacturers of ultrasonic and vibrating plate nebulizers.
Rights and permissions
About this article
Cite this article
Ferrari, F., Liu, ZH., Lu, Q. et al. Comparison of lung tissue concentrations of nebulized ceftazidime in ventilated piglets: ultrasonic versus vibrating plate nebulizers. Intensive Care Med 34, 1718–1723 (2008). https://doi.org/10.1007/s00134-008-1126-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-008-1126-4