Abstract
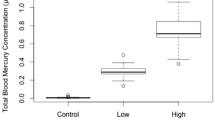
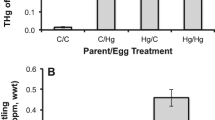
Songbirds exposed to methylmercury (MeHg) often exhibit reduced reproductive success and cognitive abilities. To better understand whether oxidative stress plays a role, we dosed zebra finches (Taeniopygia guttata) with a contaminated (1.2 ppm MeHg-cysteine) or control diet for their entire lives, including during development in the egg. Levels of antioxidant enzymes [superoxide dismutase (SOD1 and SOD2)], oxidative damage (4-hydroxynonenal; 4-HNE), and antioxidant transcription factors [nuclear factor (erythroid-derived 2)-like 2; Nrf2] were measured in the liver and pectoralis muscle of adults. MeHg treatment did not affect levels of 4-HNE or liver SOD2 or Nrf2. Birds in the MeHg treatment differed significantly from controls in pectoralis SOD1 and Nrf2, and tended to differ in liver SOD1 and pectoralis SOD2; however, we detected no overall pattern of effect of MeHg on oxidative status in dosed finches. We suspect that this is a consequence of the differential survival of MeHg-tolerant birds.
Similar content being viewed by others
References
Berntssen MHG, Aatland A, Handy RD (2003) Chronic dietary mercury exposure causes oxidative stress, brain lesions, and altered behaviour in Atlantic salmon (Salmo salar) parr. Aquat Toxicol 65:55–72. doi:10.1016/s0166-445x(03)00104-8
Cristol DA, Brasso RL, Condon AM, Fovargue RE, Friedman SL, Hallinger KK, Monroe AP, White AE (2008) The movement of aquatic mercury through terrestrial food webs. Science 320:335. doi:10.1126/science.1154082
Finger JW Jr, Hamilton MT, Glenn TC, Tuberville TD (2017) Dietary selenomethionine administration in the American alligator (Alligator mississippiensis): Hepatic and renal Se accumulation and its effects on growth and body condition. Arch Environ Contam Toxicol 72:439–448. doi:10.1007/s00244-017-0370-4
Fujimura M, Usuki F (2014) Low in situ expression of antioxidant enzymes in rat cerebellar granular cells susceptible to methylmercury. Arch Toxicol 88:109–113. doi:10.1007/s00204-013-1089-2
Fukai T, Ushio-Fukai M (2011) Superoxide dismutases: role in redox signaling, vascular function, and diseases. Antioxid Redox Signal 15:1583–1606. doi:10.1089/ars.2011.3999
Gatti R, Belletti S, Uggeri J, Vettori MV, Mutti A, Scandroglio R, Orlandini G (2004) Methylmercury cytotoxicity in PC12 cells is mediated by primary glutathione depletion independent of excess reactive oxygen species generation. Toxicology 204:175–185. doi:10.1016/j.tox.2004.06.023
Hallinger KK, Zabransky DJ, Kazmer KA, Cristol DA (2010) Birdsong differs between mercury-polluted and reference sites. Auk 127:156–161. doi:10.1525/auk.2009.09058
Hawley DM, Hallinger KK, Cristol DA (2009) Compromised immune competence in free-living tree swallows exposed to mercury. Ecotoxicology 18:499–503. doi:10.1007/s10646-009-0307-4
Henry KA, Cristol DA, Varian-Ramos CW, Bradley EL (2015) Oxidative stress in songbirds exposed to dietary methylmercury. Ecotoxicology 24:520–526. doi:10.1007/s10646-014-1400-x
Hoffman DJ, Spalding MG, Frederick PC (2005) Subchronic effects of methylmercury on plasma and organ biochemistries in great egret nestlings. Environ Toxicol Chem 24:3078–3084
Hyatt HW, Kephart WC, Holland AM, Mumford P, Mobley CB, Lowery RP, Roberts MD, Wilson JM, Kavazis AN (2016) A ketogenic diet in rodents elicits improved mitochondrial adaptations in response to resistance exercise training compared to an isocaloric Western diet. Front Physiol 7:533. doi:10.3389/fphys.2016.00533
Kenow KP, Hoffman DJ, Hines RK, Meyer MW, Bickham JW, Matson CW, Stebbins KR, Montagna P, Elfessi A (2008) Effects of methylmercury exposure on glutathione metabolism, oxidative stress, and chromosomal damage in captive-reared common loon (Gavia immer) chicks. Environ Pollut 156:732–738. doi:10.1016/j.envpol.2008.06.009
Kobiela ME, Cristol DA, Swaddle JP (2015) Risk-taking behaviours in zebra finches affected by mercury exposure. Anim Behav 103:153–160. doi:10.1016/j.anbehav.2015.02.024
Naganuma A, Miura K, Tanaka-Kagawa T, Kitahara J, Seko Y, Toyoda H, Imura N (1998) Overexpression of manganese-superoxide dismutase prevents methylmercury toxicity in HeLa cells. Life Sci 62:157–161
Ni M, Li X, Yin Z, Jiang H, Sidoryk-Wegrzynowicz M, Milatovic D, Cai J, Aschner M (2010) Methylmercury induces acute oxidative stress, altering Nrf2 protein level in primary microglial cells. Toxicol Sci 116:590–603. doi:10.1093/toxsci/kfq126
Ni M, Li X, Yin Z, Sidoryk-Wegrzynowicz M, Jiang H, Farina M, Rocha JBT, Syversen T, Aschner M (2011) Comparative study on the response of rat primary astrocytes and microglia to methylmercury toxicity. Glia 59:810–820. doi:10.1002/glia.21153
Shinyashiki M, Kumagi Y, Homma-Takeda S, Nagafune J, Takasawa N, Suzuki J, Matsuzaki I, Satoh S, Sagai M, Shimojo N (1996) Selective inhibition of the mouse brain Mn-SOD by methylmercury. Environ Toxicol Pharmacol 2:359–366
Toyama T, Sumi D, Shinkai Y, Yasutake A, Taguchi K, Tong KI, Yamamoto M, Kumagai Y (2007) Cytoprotective role of Nrf2/Keap1 system in methylmercury toxicity. Biochem Biophys Res Commun 363:645–650. doi:10.1016/j.bbrc.2007.09.017
Varian-Ramos CW, Swaddle JP, Cristol DA (2013) Familial differences in the effects of mercury on reproduction in zebra finches. Environ Pollut 182:316–323. doi:10.1016/j.envpol.2013.07.044
Varian-Ramos CW, Swaddle JP, Cristol DA (2014) Mercury reduces avian reproductive success and imposes selection: an experimental study with adult- or lifetime-exposure in zebra finch. PLoS ONE 9:e95674. doi:10.1371/journal.pone.0095674
Whitney MC, Cristol DA (2017) Impacts of sublethal mercury exposure on birds: a detailed review. Rev Environ Contam Toxicol:1–51 doi:10.1007/398_2017_4
Wolfe MF, Schwarzbach S, Sulaiman RA (1998) Effects of mercury on wildlife: a comprehensive review. Environ Toxicol Chem 17:146–160
Yee S, Choi BH (1994) Methylmercury poisoning induces oxidative stress in the mouse brain. Exp Mol Pathol 60:188–196
Yin Z, Milatovic D, Ashner JL, Syversen T, Rocha JBT, Souza DO, Sidoryk M, Albrecht J, Aschner M (2007) Methylmercury induces oxidative injury, alterations in permeability and glutamine transport in cultured astrocytes. Brain Res 1131:1–10. doi:10.1016/j.brainres.2006.10.070
Acknowledgements
Funding was provided by NSF (IOS-1257590 to John Swaddle and DAC).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Finger, J.W., Botero, J., Zhang, Y. et al. No Effect of Lifelong Methylmercury Exposure on Oxidative Status in Zebra Finches (Taeniopygia guttata): A Demonstration of Methylmercury-Induced Selection?. Bull Environ Contam Toxicol 99, 668–672 (2017). https://doi.org/10.1007/s00128-017-2202-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-017-2202-7