Abstract
Aim/hypothesis
Endoplasmic reticulum (ER) stress, which is involved in the link between inflammation and insulin resistance, contributes to the development of type 2 diabetes mellitus. In this study, we assessed whether peroxisome proliferator-activated receptor (PPAR)β/δ prevented ER stress-associated inflammation and insulin resistance in skeletal muscle cells.
Methods
Studies were conducted in mouse C2C12 myotubes, in the human myogenic cell line LHCN-M2 and in skeletal muscle from wild-type and PPARβ/δ-deficient mice and mice exposed to a high-fat diet.
Results
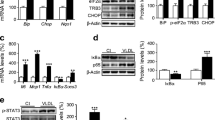
The PPARβ/δ agonist GW501516 prevented lipid-induced ER stress in mouse and human myotubes and in skeletal muscle of mice fed a high-fat diet. PPARβ/δ activation also prevented thapsigargin- and tunicamycin-induced ER stress in human and murine skeletal muscle cells. In agreement with this, PPARβ/δ activation prevented ER stress-associated inflammation and insulin resistance, and glucose-intolerant PPARβ/δ-deficient mice showed increased phosphorylated levels of inositol-requiring 1 transmembrane kinase/endonuclease-1α in skeletal muscle. Our findings demonstrate that PPARβ/δ activation prevents ER stress through the activation of AMP-activated protein kinase (AMPK), and the subsequent inhibition of extracellular-signal-regulated kinase (ERK)1/2 due to the inhibitory crosstalk between AMPK and ERK1/2, since overexpression of a dominant negative AMPK construct (K45R) reversed the effects attained by PPARβ/δ activation.
Conclusions/interpretation
Overall, these findings indicate that PPARβ/δ prevents ER stress, inflammation and insulin resistance in skeletal muscle cells by activating AMPK.






Similar content being viewed by others
Abbreviations
- 2-DG:
-
2-Deoxy-glucose
- ACC2:
-
Acetyl-CoA carboxylase 2
- AMPK:
-
AMP-activated protein kinase
- ATF6:
-
Activating transcription factor-6
- eIF2α:
-
Εukaryotic initiation factor 2α
- EMSA:
-
Electrophoretic mobility shift assay
- ER:
-
Endoplasmic reticulum
- ERK:
-
Extracellular signal-regulated kinase
- IκB:
-
Inhibitor of κB
- IRE1α:
-
Inositol-requiring 1 transmembrane kinase/endonuclease-1α
- NF-κB:
-
Nuclear factor-κB
- PERK:
-
Eukaryotic translation initiation factor-2α kinase 3
- PPAR:
-
Peroxisome proliferator-activated receptor
- UPR:
-
Unfolded protein response
- XBP1:
-
X-box binding protein-1
References
Samuel VT, Shulman GI (2012) Mechanisms for insulin resistance: common threads and missing links. Cell 148:852–871
Martin BC, Warram JH, Krolewski AS, Bergman RN, Soeldner JS, Kahn CR (1992) Role of glucose and insulin resistance in development of type 2 diabetes mellitus: results of a 25-year follow-up study. Lancet 340:925–929
Kelley DE, Goodpaster BH, Storlien L (2002) Muscle triglyceride and insulin resistance. Annu Rev Nutr 22:325–346
Hotamisligil GS (2010) Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell 140:900–917
Ron D (2002) Translational control in the endoplasmic reticulum stress response. J Clin Invest 110:1383–1388
Ozcan U, Cao Q, Yilmaz E et al (2004) Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science 306:457–461
Eizirik DL, Cardozo AK, Cnop M (2008) The role for endoplasmic reticulum stress in diabetes mellitus. Endocr Rev 29:42–61
Itani SI, Ruderman NB, Schmieder F, Boden G (2002) Lipid-induced insulin resistance in human muscle is associated with changes in diacylglycerol, protein kinase C, and IkappaB-alpha. Diabetes 51:2005–2011
Zhang K, Kaufman RJ (2008) From endoplasmic-reticulum stress to the inflammatory response. Nature 454:455–462
Zhang BB, Zhou G, Li C (2009) AMPK: an emerging drug target for diabetes and the metabolic syndrome. Cell Metab 9:407–416
Terai K, Hiramoto Y, Masaki M et al (2005) AMP-activated protein kinase protects cardiomyocytes against hypoxic injury through attenuation of endoplasmic reticulum stress. Mol Cell Biol 25:9554–9575
Dong Y, Zhang M, Wang S et al (2010) Activation of AMP-activated protein kinase inhibits oxidized LDL-triggered endoplasmic reticulum stress in vivo. Diabetes 59:1386–1396
Dong Y, Zhang M, Liang B et al (2010) Reduction of AMP-activated protein kinase alpha2 increases endoplasmic reticulum stress and atherosclerosis in vivo. Circulation 121:792–803
Wang Y, Wu Z, Li D et al (2011) Involvement of oxygen-regulated protein 150 in AMP-activated protein kinase-mediated alleviation of lipid-induced endoplasmic reticulum stress. J Biol Chem 286:11119–11131
Du J, Guan T, Zhang H, Xia Y, Liu F, Zhang Y (2008) Inhibitory crosstalk between ERK and AMPK in the growth and proliferation of cardiac fibroblasts. Biochem Biophys Res Commun 368:402–407
Hwang SL, Jeong YT, Li X et al (2013) Inhibitory cross-talk between the AMPK and ERK pathways mediates endoplasmic reticulum stress-induced insulin resistance in skeletal muscle. Br J Pharmacol 169:69–81
Michalik L, Auwerx J, Berger JP et al (2006) International Union of Pharmacology. LXI. Peroxisome proliferator-activated receptors. Pharmacol Rev 58:726–741
Lee CH, Chawla A, Urbiztondo N et al (2003) Transcriptional repression of atherogenic inflammation: modulation by PPARdelta. Science 302:453–457
Pascual G, Fong AL, Ogawa S et al (2005) A SUMOylation-dependent pathway mediates transrepression of inflammatory response genes by PPAR-gamma. Nature 437:759–763
Daynes RA, Jones DC (2002) Emerging roles of PPARs in inflammation and immunity. Nat Rev Immunol 2:748–759
Devchand PR, Keller H, Peters JM, Vazquez M, Gonzalez FJ, Wahli W (1996) The PPARalpha-leukotriene B4 pathway to inflammation control. Nature 384:39–43
Auwerx J, Baulieu E, Beato M et al (1999) A unified nomenclature system for the nuclear receptor superfamily. Cell 97:161–163
Schuler M, Ali F, Chambon C et al (2006) PGC1alpha expression is controlled in skeletal muscles by PPARbeta, whose ablation results in fiber-type switching, obesity, and type 2 diabetes. Cell Metab 4:407–414
Lee CH, Olson P, Hevener A et al (2006) PPARdelta regulates glucose metabolism and insulin sensitivity. Proc Natl Acad Sci U S A 103:3444–3449
Barish GD, Narkar VA, Evans RM (2006) PPAR delta: a dagger in the heart of the metabolic syndrome. J Clin Invest 116:590–597
Krämer DK, Al-Khalili L, Guigas B, Leng Y, Garcia-Roves PM, Krook A (2007) Role of AMP kinase and PPARdelta in the regulation of lipid and glucose metabolism in human skeletal muscle. J Biol Chem 282:19313–19320
Salvadó L, Coll T, Gómez-Foix AM et al (2013) Oleate prevents saturated-fatty-acid-induced ER stress, inflammation and insulin resistance in skeletal muscle cells through an AMPK-dependent mechanism. Diabetologia 56:1372–1382
Nadra K, Anghel SI, Joye E et al (2006) Differentiation of trophoblast giant cells and their metabolic functions are dependent on peroxisome proliferator-activated receptor beta/delta. Mol Cell Biol 26:3266–3281
Oliver WR Jr, Shenk JL, Snaith MR et al (2001) A selective peroxisome proliferator-activated receptor delta agonist promotes reverse cholesterol transport. Proc Natl Acad Sci U S A 98:5306–5311
Kino T, Rice KC, Chrousos GP (2007) The PPARβ/δ agonist GW501516 suppresses interleukin 6-mediated hepatocyte acute phase reaction via STAT3 inhibition. Eur J Clin Invest 37:425–433
Barroso E, Rodríguez-Calvo R, Serrano-Marco L et al (2011) The PPARβ/δ activator GW501516 prevents the down-regulation of AMPK caused by a high-fat diet in liver and amplifies the PGC-1α-Lipin 1-PPARα pathway leading to increased fatty acid oxidation. Endocrinology 152:1848–1859
Xu C, Bailly-Maitre B, Reed JC (2005) Endoplasmic reticulum stress: cell life and death decisions. J Clin Invest 115:2656–2664
Weigert C, Brodbeck K, Staiger H et al (2004) Palmitate, but not unsaturated fatty acids, induces the expression of interleukin-6 in human myotubes through proteasome-dependent activation of nuclear factor-kappaB. J Biol Chem 279:23942–23952
Kern PA, Ranganathan S, Li C, Wood L, Ranganathan G (2001) Adipose tissue tumor necrosis factor and interleukin-6 expression in human obesity and insulin resistance. Am J Physiol Endocrinol Metab 280:E745–E751
Pickup JC, Mattock MB, Chusney GD, Burt D (1997) NIDDM as a disease of the innate immune system: association of acute-phase reactants and interleukin-6 with metabolic syndrome X. Diabetologia 40:1286–1292
Hage Hassan R, Hainault I, Vilquin JT et al (2012) Endoplasmic reticulum stress does not mediate palmitate-induced insulin resistance in mouse and human muscle cells. Diabetologia 55:204–214
Liang CP, Han S, Okamoto H et al (2004) Increased CD36 protein as a response to defective insulin signaling in macrophages. J Clin Invest 113:764–773
Mu J, Brozinick JT, Valladares O, Bucan M, Birnbaum MJ (2001) A role for AMP-activated protein kinase in contraction- and hypoxia-regulated glucose transport in skeletal muscle. Mol Cell 7:1085–1094
Andrulionyte L, Peltola P, Chiasson JL, Laakso M, STOP-NIDDM Study Group (2006) Single nucleotide polymorphisms of PPARD in combination with the Gly482Ser substitution of PGC-1A and the Pro12Ala substitution of PPARG2 predict the conversion from impaired glucose tolerance to type 2 diabetes: the STOP-NIDDM trial. Diabetes 55:2148–2152
Coll T, Alvarez-Guardia D, Barroso E et al (2010) Activation of peroxisome proliferator-activated receptor-δ by GW501516 prevents fatty acid-induced nuclear factor-κB activation and insulin resistance in skeletal muscle cells. Endocrinology 151:1560–1569
Cao M, Tong Y, Lv Q et al (2012) PPARδ Activation RESCUES PAncreatic β-cell line INS-1E from palmitate-induced endoplasmic reticulum stress through enhanced fatty acid oxidation. PPAR Res 2012:680684
Ramirez T, Tong M, Chen WC, Nguyen QG, Wands JR, de la Monte SM (2013) Chronic alcohol-induced hepatic insulin resistance and endoplasmic reticulum stress ameliorated by peroxisome-proliferator activated receptor-δ agonist treatment. J Gastroenterol Hepatol 28:179–187
Bojic LA, Burke AC, Chokker SS et al (2014) Peroxisome Proliferator-Activated Receptor d agonist GW1516 attenuates diet-induced aortic inflammation, insulin resistance, and atherosclerosis in low-density lipoprotein receptor knockout mice. Arterioscler Thromb Vasc Biol 34:52–60
Jager J, Corcelle V, Grémeaux T et al (2011) Deficiency in the extracellular signal-regulated kinase 1 (ERK1) protects leptin-deficient mice from insulin resistance without affecting obesity. Diabetologia 54:180–189
Acknowledgements
We thank A. Orozco (Department of Biochemistry and Molecular Biology of the University of Barcelona, Spain) for experimental assistance with human myotube cultures. We thank M. J. Birnbaum (Howard Hughes Medical Institute, University of Pennsylvania, Philadelphia, PA, USA) for the pcDNA3/pAMPKalpha2-K45R plasmid.
Funding
This study was partly supported by funds from the Spanish Ministerio de Economía y Competitividad (SAF2009-06939 and SAF2012-30708) and the European Union ERDF. CIBER de Diabetes y Enfermedades Metabólicas Asociadas (CIBERDEM) is an Instituto de Salud Carlos III project. LS was supported by an FPI grant from the Spanish Ministerio de Economía y Competitividad. We thank the University of Barcelona’s Language Advisory Service for revising the manuscript.
Contribution statement
LS, EB, AMG-F, XP, LM, WW and MV-C processed the samples, analysed and prepared the data and were involved in drafting the article. LS, EB, AG-F, XP, LM and WW contributed to the interpretation of the data and revised the article. MV-C and LS designed the experiments and analysed and interpreted the data. MV-C wrote the manuscript and is responsible for the integrity of the work as a whole. All authors approved the final version of the manuscript.
Duality of interest
The authors declare that there is no duality of interest associated with this manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM Methods
(PDF 75 kb)
ESM Table 1
(PDF 43 kb)
Rights and permissions
About this article
Cite this article
Salvadó, L., Barroso, E., Gómez-Foix, A.M. et al. PPARβ/δ prevents endoplasmic reticulum stress-associated inflammation and insulin resistance in skeletal muscle cells through an AMPK-dependent mechanism. Diabetologia 57, 2126–2135 (2014). https://doi.org/10.1007/s00125-014-3331-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-014-3331-8