Abstract
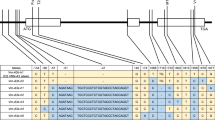
The sequence data from 5′ UTR, intronic, coding and 3′ UTR regions of Ppd-A1 and Ppd-B1 were investigated for a total of 158 accessions of emmer wheat landraces comprising 19 of wild emmer wheat (Triticum dicoccoides), 45 of hulled emmer wheat (T. dicoccum) and 94 of free-threshing (FT) emmer wheat (T. durum etc.). We detected some novel types of deletions in the coding regions from 22 hulled emmer accessions and 20 FT emmer accessions. Emmer wheat accessions with these deletions could produce predicted proteins likely to lack function. We also observed some novel mutations in Ppd-B1. Sixty-seven and forty-one haplotypes were found in Ppd-A1 and Ppd-B1, respectively. Some mutations found in this study have not been known, so they have potential for useful genetic resources for wheat breeding. On the basis of sequence data from the 5′ UTR region, both Ppd-A1 and Ppd-B1 haplotypes were divided into two groups (Type AI/AII and Type BI/BII). Types AI and AII of Ppd-A1 suggested gene flow between wild and hulled emmer. On the other hand, Types BI and BII of Ppd-B1 suggested gene flow between wild and FT emmer. More than half of hulled emmer accessions were Type AII/BI but few FT emmer accessions were of this type. Therefore, over half of the hulled emmer did not contribute to evolution of FT emmer.






Similar content being viewed by others
References
Beales J, Turner A, Griffiths S, Snape JW, Laurie DA (2007) A pseudo-response regulator is misexpressed in the photoperiod insensitive ppd-D1a mutant of wheat (Triticum aestivum L.). TAG. Theor Appl Genet 115(5):721–733
Bentley A, Turner A, Gosman N, Leigh F, Maccaferri M, Dreisigacker S et al (2010) Frequency of photoperiod-insensitive Ppd-A1a alleles in tetraploid, hexaploid and synthetic hexaploid wheat germplasm. Plant Breed 130:10–15
Dubcovsky J, Dvorak J (2007) Genome plasticity a key factor in the success of polyploid wheat under domestication. Science 316(5833):1862
Escaravage N, Questiau S, Pornon A, Doche B, Taberlet P (1998) Clonal diversity in a Rhododendron ferrugineum L. (ericaceae) population inferred from AFLP markers. Mol Ecol 7(8):975–982
Griffiths S, Dunford RP, Coupland G, Laurie DA (2003) The evolution of CONSTANS-like gene families in barley, rice, and arabidopsis. Plant Physiol 131(4):1855
Guo Z, Song Y, Zhou R, Ren Z, Jia J (2010) Discovery, evaluation and distribution of haplotypes of the wheat ppd-D1 gene. New Phytol 185(3):841–851
Hall TA (1999). BioEdit: a user-friendly biological sequence alignment editor and analysis program for windows 95/98/NT.41, 95–98
Kato K, Yamagata H (1988) Method for evaluation of chilling requirement and narrow-sense earliness of wheat cultivars. Ikushugaku Zasshi 38(2):172–186
Kato K, Yokoyama H (1992) Geographical variation in heading characters among wheat landraces, Triticum aestivum L., and its implication for their adaptability. Theor Appl Genet 84(3):259–265
Kihara H (1944) Discovery of the DD-analyser, one of the ancestors of Triticum vulgare. Agric. Hortic 19:13–14
Kimura M (1980) A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol 16(2):111–120
Kuchel H, Hollamby G, Langridge P, Williams K, Jefferies SP (2006) Identification of genetic loci associated with ear-emergence in bread wheat. Theor Appl Genet 113(6):1103–1112
Law C, Worland A (1997) Genetic analysis of some flowering time and adaptive traits in wheat. New Phytol 137:19–28
Librado P, Rozas J (2009) DnaSP v5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics 25(11):1451
Luo MC, Yang ZL, You FM, Kawahara T, Waines JG, Dvorak J (2007) The structure of wild and domesticated emmer wheat populations, gene flow between them, and the site of emmer domestication. Theor Appl Genet 114:947–959
McFadden E, Sears E (1944) The artificial synthesis of triticum spelta. Rec Genet Soc Am 13:26–27
Mizuno T, Nakamichi N (2005) Pseudo-response regulators (PRRs) or true oscillator components (TOCs). Plant Cell Physiol 46(5):677
Mohler V, Lukman R, Ortiz-Islas S, William M, Worland AJ, van Beem J et al (2004) Genetic and physical mapping of photoperiod insensitive gene ppd-B1 in common wheat. Euphytica 138(1):33–40
Motzo R, Giunta F (2007) The effect of breeding on the phenology of Italian durum wheats: from landraces to modern cultivars. Eur J Agron 26(4):462–470
Nakamichi N, Kita M, Niinuma K, Ito S, Yamashino T, Mizoguchi T et al (2007) Arabidopsis clock-associated pseudo-response regulators PRR9, PRR7 and PRR5 coordinately and positively regulate flowering time through the canonical CONSTANS-dependent photoperiodic pathway. Plant Cell Physiol 48(6):822
Nei M (1987) Molecular evolutionary genetics. Columbia University Press, New York
Nei M, Miller J (1990) A simple method for estimating average number of nucleotide substitutions within and between populations from restriction data. Genetics 125(4):873
Nesbitt M, Samuel D (1998) Wheat domestication: archaeobotanical evidence. Science 279(5356):1433
Ozkan H, Willcox G, Graner A, Salamini F, Kilian B (2011) Geographic distribution and domestication of wild emmer wheat (Triticum dicoccoides). Genet Resour Crop Evol 58:11–53
Pecetti L, Damania AB (1996) Geographic variation in tetraploid wheat (Triticum turgidum spp. turgidum convar. durum) landraces from two provinces in Ethiopia. Genet Resour Crop Evol 43(5):395–407
Pecetti L, Annicchiarico P, Damania AB (1992) Biodiversity in a germplasm collection of durum wheat. Euphytica 60(3):229–238
Rozen S, Skaletsky H (2000) Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol 132(3):365–386
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4(4):406
Shewry P (2009) Wheat. J Exp Bot 60(6):1537
Sourdille P, Snape J, Cadalen T, Charmet G, Nakata N, Bernard S et al (2000) Detection of QTLs for heading time and photoperiod response in wheat using a doubled-haploid population. Genome 43(3):487–494
Takenaka S, Mori N, Kawahara T (2010) Genetic variation in domesticated emmer wheat (Triticum turgidum L.) in and around abyssinian highlands. Breed Sci 60(3):212–227
Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol Biol Evol 24(8):1596
Tanio M, Kato K (2007) Development of near-isogenic lines for photoperiod-insensitive genes, ppd-B1 and ppd-D1, carried by the Japanese wheat cultivars and their effect on apical development. Breed Sci 57(1):65–72
Tanno K, Willcox G (2006) How fast was wild wheat domesticated? Science 311(5769):1886
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22(22):4673
Turner A, Beales J, Faure S, Dunford RP, Laurie DA (2005) The pseudo-response regulator Ppd-H1 provides adaptation to photoperiod in barley. Science’s STKE 310(5750):1031
Wilhelm EP, Turner AS, Laurie DA (2009) Photoperiod insensitive Ppd-A1a mutations in tetraploid wheat (Triticum durum desf.). TAG Theor Appl Genet 118(2):285–294
Worland A, Law C (1986) Genetic analysis of chromosome 2D of wheat. I. The location of genes affecting height, day-length insensitivity, hybrid dwarfism and yellow-rust resistance. Z.PFLANZENZUECHT 96(4):331–345
Yan L, Loukoianov A, Tranquilli G, Helguera M, Fahima T, Dubcovsky J (2003) Positional cloning of the wheat vernalization gene VRN1. Proc Nat Acad Sci 100(10):6263
Yan L, Loukoianov A, Blechl A, Tranquilli G, Ramakrishna W, SanMiguel P et al (2004) The wheat VRN2 gene is a flowering repressor down-regulated by vernalization. Science 303(5664):1640
Yan L, Fu D, Li C, Blechl A, Tranquilli G, Bonafede M et al (2006) The wheat and barley vernalization gene VRN3 is an orthologue of FT. Proc Nat Acad Sci 103(51):19581
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by T. Komatsuda.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Takenaka, S., Kawahara, T. Evolution and dispersal of emmer wheat (Triticum sp.) from novel haplotypes of Ppd-1 (photoperiod response) genes and their surrounding DNA sequences. Theor Appl Genet 125, 999–1014 (2012). https://doi.org/10.1007/s00122-012-1890-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00122-012-1890-y