Abstract
NLRP3 inflammasome activation in the retinal pigment epithelium (RPE) is observed in atrophic age-related macular degeneration (AMD), and pharmacological NLRP3 inhibition may provide a therapeutic strategy to halt disease progression. We tested selective NLRP3 inhibitors (IFM-514, IFM-632, and CRID3) for their efficacy in human and murine RPE cells. Inflammasome activation was induced in primary human RPE cells and ARPE-19 cells following priming with IL-1α by different stimuli, including lysosomal membrane permeabilization by leucyl-leucine methyl ester (Leu-Leu-OMe), oxidative damage induced by hydrogen peroxide, lipofuscin-mediated photooxidative damage induced by incubation with 4-hydroxynonenal-modified photoreceptor outer segments and subsequent blue light irradiation, and P2X7 receptor activation by benzoylbenzoyl-ATP. Independent of the applied activation mechanism, treatment with the NLRP3 inhibitors IFM-632, IFM-514, and CRID3 resulted in a significant suppression of inflammasome activation as assessed by IL-1β and LDH release. Likewise, inflammasome activation in blue light-irradiated Abca4−/− mouse and Leu-Leu-OMe-treated wild-type mouse RPE/choroid/sclera eye cups was significantly reduced by treatment with the NLRP3 inhibitors. These results indicate that the investigated selective NLRP3 inhibitors are effective in human and murine RPE cells, thus representing promising agents for the future evaluation of inflammasome inhibition as a therapeutic strategy in atrophic AMD.
Key messages
• NLRP3 inhibitors suppress inflammasome activation in human RPE cells independent of trigger.
• Light-induced inflammasome activation in Abca4−/− mouse eye cups is reduced by NLRP3 inhibitors.
• Novel selective NLRP3 inhibitors are effective in human and murine RPE cells.
• Promising compounds for pharmaceutical intervention in atrophic AMD.






Similar content being viewed by others
References
European Society of Retina Specialists (EURETINA) (2017) Retinal diseases in Europe: prevalence, incidence and healthcare needs. Available online at http://www.euretina.org/downloads/EURETINA_Retinal_Diseases.pdf. Accessed 31 Aug 2018
Lim LS, Mitchell P, Seddon JM, Holz FG, Wong TY (2012) Age-related macular degeneration. Lancet 379:1728–1738
Tarallo V, Hirano Y, Gelfand BD, Dridi S, Kerur N, Kim Y, Cho WG, Kaneko H, Fowler BJ, Bogdanovich S et al (2012) DICER1 loss and Alu RNA induce age-related macular degeneration via the NLRP3 inflammasome and MyD88. Cell 149:847–859
Doyle SL, Campbell M, Ozaki E, Salomon RG, Mori A, Kenna PF, Farrar GJ, Kiang AS, Humphries MM, Lavelle EC et al (2012) NLRP3 has a protective role in age-related macular degeneration through the induction of IL-18 by drusen components. Nat Med 18:791–798
Tseng WA, Thein T, Kinnunen K, Lashkari K, Gregory MS, D’Amore PA, Ksander BR (2013) NLRP3 inflammasome activation in retinal pigment epithelial cells by lysosomal destabilization: implications for age-related macular degeneration. Invest Ophthalmol Vis Sci 54:110–120
Cao S, Wang JC, Gao J, Wong M, To E, White VA, Cui JZ, Matsubara JA (2016) CFH Y402H polymorphism and the complement activation product C5a: effects on NF-κB activation and inflammasome gene regulation. Br J Ophthalmol 100:713–718
Ijima R, Kaneko H, Ye F, Nagasaka Y, Takayama K, Kataoka K, Kachi S, Iwase T, Terasaki H (2014) Interleukin-18 induces retinal pigment epithelium degeneration in mice. Invest Ophthalmol Vis Sci 55:6673–6678
Budiene B, Liutkeviciene R, Gustiene O, Ugenskiene R, Laukaitiene D, Savukaityte A, Vilkeviciute A, Steponaviciute R, Rocyte A, Zaliuniene D (2018) The association of matrix metalloproteinases polymorphisms and interleukins in advanced age-related macular degeneration. Ophthalmic Genet 39:463–472
Zhao M, Bai Y, Xie W, Shi X, Li F, Yang F, Sun Y, Huang L, Li X (2015) Interleukin-1β level is increased in vitreous of patients with neovascular age-related macular degeneration (nAMD) and polypoidal choroidal vasculopathy (PCV). PLoS One 10:1–10
Gao J, Cui JZ, To E, Cao S, Matsubara JA (2018) Evidence for the activation of pyroptotic and apoptotic pathways in RPE cells associated with NLRP3 inflammasome in the rodent eye. J Neuroinflammation 15:15
Brandstetter C, Patt J, Holz FG, Krohne TU (2016) Inflammasome priming increases retinal pigment epithelial cell susceptibility to lipofuscin phototoxicity by changing the cell death mechanism from apoptosis to pyroptosis. J Photochem Photobiol B 161:177–183
Akhtar-Schäfer I, Wang L, Krohne TU, Xu H, Langmann T (2018) Modulation of three key innate immune pathways for the most common retinal degenerative diseases. EMBO Mol Med 10:e8259
Heneka MT, McManus RM, Latz E (2018) Inflammasome signalling in brain function and neurodegenerative disease. Nat Rev Neurosci 19:610–621
Latz E, Xiao TS, Stutz A (2013) Activation and regulation of the inflammasomes. Nat Rev Immunol 13:397–411
Karmakar M, Katsnelson M, Malak HA, Greene NG, Howell SJ, Hise AG, Camilli A, Kadioglu A, Dubyak GR, Pearlman E (2018) Neutrophil IL-1 β processing induced by pneumolysin is mediated by the NLRP3/ASC inflammasome and caspase-1 activation and is dependent on K+ efflux. J Immunol 194:1763–1775
Liu RT, Wang A, To E, Gao J, Cao S, Cui JZ, Matsubara JA (2015) Vinpocetine inhibits amyloid-beta induced activation of NF-κB, NLRP3 inflammasome and cytokine production in retinal pigment epithelial cells. Exp Eye Res 127:49–58
Brandstetter C, Mohr LKM, Latz E, Holz FG, Krohne TU (2015) Light induces NLRP3 inflammasome activation in retinal pigment epithelial cells via lipofuscin-mediated photooxidative damage. J Mol Med 93:905–916
Yang D, Elner SG, Clark AJ, Hughes BA, Petty HR, Elner VM (2011) Activation of P2X receptors induces apoptosis in human retinal pigment epithelium. Invest Opthalmol Vis Sci 52:1522–1530
IFM Therapeutics (2017) Compounds and compositions for treating conditions associated with NLRP activity. World Intellectual Property Organization (WIPO), Geneva, Switzerland; publication number WO/2017/184624. Available online at https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2017184624. Accessed 31 Aug 2018
Coll RC, Robertson AAB, Chae JJ, Higgins SC, Muñoz-Planillo R, Inserra MC, Vetter I, Dungan LS, Monks BG, Stutz A et al (2015) A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat Med 21:248–255
Primiano MJ, Lefker BA, Bowman MR, Bree AG, Hubeau C, Bonin PD, Mangan M, Dower K, Monks BG, Cushing L et al (2018) Efficacy and pharmacology of the NLRP3 inflammasome inhibitor CP-456,773 (CRID3) in murine models of dermal and pulmonary. Inflammation 773:2421–2433
Krohne TU, Stratmann NK, Kopitz J, Holz FG (2010) Effects of lipid peroxidation products on lipofuscinogenesis and autophagy in human retinal pigment epithelial cells. Exp Eye Res 90:465–471
Krohne TU, Kaemmerer E, Holz FG (2010) Lipid peroxidation products reduce lysosomal protease activities in human retinal pigment epithelial cells via two different mechanisms of action. Exp Eye Res 90:261–266
Charbel Issa P, Barnard AR, Singh MS, Carter E, Jiang Z, Radu RA, Schraermeyer U, MacLaren RE (2013) Fundus autofluorescence in the Abca4 −/− mouse model of Stargardt disease—correlation with accumulation of A2E, retinal function, and histology. Invest Ophthalmol Vis Sci 54:5602–5612
Weng J, Mata NL, Azarian SM, Tzekov RT, Birch DG, Travis GH (1999) Insights into the function of rim protein in photoreceptors and etiology of Stargardt’s disease from the phenotype in abcr knockout mice. Cell 98:13–23
Kanneganti TD, Ozoren N, Body-Malapel M, Amer A, Park JH, Franchi L, Whitfield J, Barchet W, Colonna M, Vandenabeele P et al (2006) Bacterial RNA and small antiviral compounds activate caspase-1 through cryopyrin/Nalp3. Nature 440:233–236
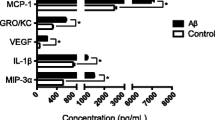
Mohr LK, Hoffmann AV, Brandstetter C, Holz FG, Krohne TU (2015) Effects of inflammasome activation on secretion of inflammatory cytokines and vascular endothelial growth factor by retinal pigment epithelial cells. Investig Opthalmol Vis Sci 56:6404
Dutot M, Liang H, Pauloin T, Brignole-Baudouin F, Baudouin C, Warnet JM, Rat P (2008) Effects of toxic cellular stresses and divalent cations on the human P2X7 cell death receptor. Mol Vis 14:889–897
Katsnelson MA, Rucker LG, Russo HM, Dubyak GR (2015) K+ efflux agonists induce NLRP3 inflammasome activation independently of Ca2+ signaling. J Immunol 194:3937–3952
Guha S, Baltazar GC, Coffey EE, Tu LA, Lim JC, Beckel JM, Patel S, Eysteinsson T, Lu W, O’Brien-Jenkins A et al (2013) Lysosomal alkalinization, lipid oxidation, and reduced phagosome clearance triggered by activation of the P2X7 receptor. FASEB J 27:4500–4509
Nalbandian A, Khan AA, Srivastava R, Llewellyn KJ, Tan B, Shukr N, Fazli Y, Kimonis VE, BenMohamed L (2017) Activation of the NLRP3 inflammasome is associated with Valosin-containing protein myopathy. Inflammation 40:21–41
Ludwig-portugall I, Bartok E, Dhana E, Evers BD, Primiano MJ, Hall JP, Franklin BS, Knolle PA, Hornung V, Hartmann G et al (2016) An NLRP3-specific inflammasome inhibitor attenuates crystal-induced kidney fibrosis in mice. Kidney Int 90:525–539
Dempsey C, Rubio Araiz A, Bryson KJ, Finucane O, Larkin C, Mills EL, Robertson AAB, Cooper MA, O’Neill LAJ, Lynch MA (2017) Inhibiting the NLRP3 inflammasome with MCC950 promotes non-phlogistic clearance of amyloid-β and cognitive function in APP/PS1 mice. Brain Behav Immun 61:306–316
Zhou R, Yazdi AS, Menu P (2011) A role for mitochondria in NLRP3 inflammasome activation. Nature 469:221–225
Giuliani AL, Sarti AC, Falzoni S, Di Virgilio F (2017) The P2X7 receptor-interleukin-1 liaison. Front Pharmacol 8:1–10
Arulkumaran N, Unwin RJ, Tam FWK (2011) A potential therapeutic role for P2X7 receptor (P2X7R) antagonists in the treatment of inflammatory diseases. Expert Opin Investig Drugs 20:897–915
Di Virgilio F, Ben DD, Sarti AC, Giuliani AL, Falzoni S (2017) The P2X7 receptor in infection and inflammation. Immunity 47:15–31
Kerur N, Hirano Y, Tarallo V, Fowler BJ, Bastos-Carvalho A, Yasuma T, Yasuma R, Kim Y, Hinton DR, Kirschning CJ et al (2013) TLR-independent and P2X7-dependent signaling mediate Alu RNA-induced NLRP3 inflammasome activation in geographic atrophy. Invest Ophthalmol Vis Sci 54:3–9
Notomi S, Hisatomi T, Murakami Y, Terasaki H, Sonoda S, Asato R, Takeda A, Ikeda Y, Enaida H, Sakamoto T et al (2013) Dynamic increase in extracellular ATP accelerates photoreceptor cell apoptosis via ligation of P2RX7 in subretinal hemorrhage. PLoS One 8:e53338
Notomi S, Hisatomi T, Kanemaru T, Takeda A, Ikeda Y, Enaida H, Kroemer G, Ishibashi T (2011) Critical involvement of extracellular ATP acting on P2RX7 purinergic receptors in photoreceptor cell death. Am J Pathol 179:2798–2809
Acknowledgements
NLRP3 inhibitors IFM-632 and IFM-514 were kindly provided by IFM Therapeutics (Boston, Massachusetts, USA). Abca4−/− mice were a kind gift from Alun Barnard (University of Oxford, Oxford, UK).
Funding
This work was supported by the China Scholarship Council (CSC; to L.W.), Ernst and Berta Grimmke Foundation (to P.P.L. and T.U.K.), German Research Foundation (DFG; grant KR 2863/7-2 to T.U.K., collaborative research centers SFB/TRR57 and SFB/TRR83), Volker Homann Foundation (to T.U.K.), Dr. Eberhard und Hilde Rüdiger Foundation (to T.U.K.), and the European Research Council (ERC) grant InflammAct (to E.L.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
L. Wang, S. Schmidt, P. P. Larsen declare that they have no conflict of interest. J. H. Meyer declares support from Heidelberg Engineering. W. R. Roush is a shareholder and employee of IFM Therapeutics. E. Latz is a co-founder and consultant of IFM Therapeutics. F. G. Holz declares support from Acucela, Allergan, Bayer, Centervue, NightstarX, Novartis, Genentech/Roche, Heidelberg Engineering, Optos, and Carl Zeiss Meditec. T. U. Krohne declares support from Alimera Sciences, Bayer, Heidelberg Engineering, and Novartis.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, L., Schmidt, S., Larsen, P.P. et al. Efficacy of novel selective NLRP3 inhibitors in human and murine retinal pigment epithelial cells. J Mol Med 97, 523–532 (2019). https://doi.org/10.1007/s00109-019-01753-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-019-01753-5