Abstract
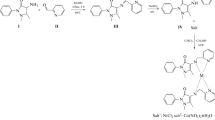
Several hydroxyl Schiff base (HSB) compounds (1–10) with good radical scavenging activity (RSA) were designed. Compounds 6, 7, and 10 showed better RSAs than the common synthetic antioxidant 2,6-diter-butyl-4-methylphenol (BHT) in DPPH• and ABTS• assays. To probe whether these HSB compounds may exert their antioxidant effect through transition metal ion chelation, the copper and ferrous chelating abilities of them were investigated. It was found by fluorescence quenching spectra that the binding constants K a were in the range of 0.85×103–7.30×104 M−1. Further study was carried out by the complexation of a representative compound 5 with ferrous ion in mass spectrum. A 2:1 5-ferrous complex was readily formed in a methanol–water solution (v:v, 8:2), which confirmed that the chelation happened when the HSB compounds were treated with transition metal ions. The above results indicated that the transition metal ion chelation play an important role in their antioxidant abilities.





Similar content being viewed by others
References
Eftink MR (1991) Fluorescence quenching reactions. In: Dewey TG (ed) Biophysical and biochemical aspects of fluorescence spectroscopy. Plenum Press, New York, pp 1–44
Halliwell B, Gutteridge JMC (1999) Free radicals in biology and medicine. Oxford University Press, Oxford
Hipkiss AR (2005) Glycation, ageing and carnosine: are carnivorous diets beneficial. Mech Ageing Dev 126:1034–1039
Lakowica JR, Weber G (1973) Quenching of fluorescence by oxygen. A probe for structural fluctuations in macromolecules. Biochemistry 12:4161–4170
Lakowicz JR (1999) Principles of fluorescence spectroscopy. Plenum Press, New York
Lee YL, Yen MT, Mau JL (2007) Antioxidant properties of various extracts from Hypizigus marmoreus. Food Chem 104:1–9
Maurice RE, Camillo AG (1981) Fluorescence quenching studies with proteins. Anal Biochem 114:199–212
Pan YM, Zhu JC, Wang HS, Zhang XP, Zhang Y, He CH, Ji XW, Li HY (2007) Antioxidant activity of ethanolic extract of Cortex fraxini and use in peanut oil. Food Chem 103:913–918
Pan YM, He CH, Wang HS, Ji XW, Wang K, Liu PZ (2010) Antioxidant activity of microwave-assisted extract of Buddleia officinalis and its major active component. Food Chem 121:497–502
Papadopoulou A, Green RJ, Frazier RA (2005) Interaction of flavonoids with bovine serum albumin: a fluorescene quenching study. J Agric Food Chem 53:158–163
Perez CA, Wei Y, Guo M (2009) Iron-binding and anti-Fenton properties of baicalein and baicalin. J Inorg Biochem 103:326–332
Tang YZ, Liu ZQ (2007) Free-radical-scavenging effect of carbazole derivatives on DPPH and ABTS radicals. Cell Biochem Funct 25:149–158
Wang HX, Tang YZ, Liu ZQ (2007) Ability of Schiff bases with hydroxyl-substituent to scavenge radicals. Chin J Synth Chem 24:1105–1108
Acknowledgments
This study was supported by the National Natural Science Foundation of China (No. 20762001), the Project of the Key Laboratory of Medicinal Chemical Resources and Molecular Engineering, Guangxi Normal University, China (No.0630006-5D09), and the Guangxi Department of Education research project (No. 200807MS075, 200807MS076, 200911MS281, 200911MS282).
Author information
Authors and Affiliations
Corresponding authors
Appendix: synthesis of the derivatives
Appendix: synthesis of the derivatives
Synthesis
General procedure for the preparation of 1–10: the mixture of aminophenol (5 mmol), aromatic aldehydes (6 mmol), ethanol (10 ml), and triethylamine (0.1 mmol) was refluxed at 90°C for 2 h and then filtered to give crystal or powder of HSB derivatives.
Spectra data for compounds 1–10
Compound 1: 1H NMR (DMSO, 500HZ) δ: 9.50 (s, 1H, OH), 8.62 (s, 1H, N=CH), 7.90 (d, J = 7.67 Hz, 2H, Ar-H), 7.49–7.51 (m, 3H, Ar-H), 7.21 (d, J = 8.57 Hz, 2H, Ar-H), 6.82 (d, J = 8.57 Hz, 2H, Ar-H). EMS: m/z: 198 [M + H]+.
Compound 2: 1H NMR (DMSO, 500HZ) δ: 8.29 (d, J = 7.20 Hz, 1H, N=CH), 7.90 (d, J = 8.50 Hz, 1H, Ar-H), 7.68 (dd, J = 8.79 Hz, 5.5 Hz, 2H, Ar-H), 7.62 (t, J = 7.26 Hz, 1H, OH), 7.51 (d, J = 7.38 Hz, 1H, Ar-H), 7.37 (d, J = 7.27 Hz, 1H, Ar-H), 7.29 (s, 2H, Ar-H), 6.83 (t, J = 7.30 Hz, 1H, Ar-H), 6.65 (t, J = 7.57 Hz, 1H, Ar-H), 6.18 (d, J = 7.5 Hz, 1H, Ar-H). EMS: m/z: 198 [M + H]+
Compound 3: 1H NMR (DMSO,500HZ) δ: 13.12 (s, 1H, OH), 9.64 (s, 1H, OH), 8.91 (s, 1H, N=CH), 7.67 (d, J = 7.53 Hz, 1H, Ar-H), 7.42 (d, J = 7.75 Hz, 1H, Ar-H), 7.25 (d, J = 7.86 Hz, 1H, Ar-H), 6.96 (d, J = 7.47 Hz, 2H, Ar-H), 6.84 (d, J = 7.65 Hz, 1H, Ar-H), 6.78–6.74 (m, 2H, Ar-H). EMS: m/z: 214 [M+H]+.
Compound 4: 1H NMR (DMSO,500HZ) δ: 13.41 (s, 1H, OH), 9.67 (s, 1H, OH), 8.91 (s, 1H, N=CH), 7.60 (d, J = 8.11 Hz, 1H, Ar-H), 7.32-7.39 (m, 3H, Ar-H), 6.93-6.98 (m, 2H, Ar-H), 6.85 (d, J = 8.6 Hz, 2H, Ar-H). EMS: m/z: 214 [M + H]+.
Compound 5: 1H NMR (DMSO,500HZ) δ: 13.76 (s, 1H, OH), 9.71 (s, 1H, OH), 8.97 (s, 1H, N=CH), 7.62 (d, J = 7.50 Hz, 1H, Ar-H), 7.36-7.41 (m, 2H, Ar-H), 7.14 (t, J = 7.50 Hz, 1H, Ar-H), 6.87–6.98 (m, 4H, Ar-H). EMS: m/z: 214 [M + H]+.
Compound 6: 1H NMR (DMSO,500HZ) δ: 9.42 (1s, 1H, OH), 8.58 (s, 1H, N=CH), 7.82 (d, 2H, J = 8.84Hz, Ar-H), 7.28 (d, J = 7.87Hz, 1H, Ar-H), 7.14 (d, J = 7.94Hz, 1H, Ar-H), 7.01 (d, J = 8.16Hz, 1H, Ar-H), 6.91 (d, J = 7.5Hz, 1H, Ar-H), 6.78 (d, J = 8.9Hz, 2H, Ar-H), 3.01 (s, 6H, CH3). EMS: m/z: 241 [M + H]+
Compound 7: 1H NMR (DMSO, 500HZ) δ: 9.31 (1s, 1H, OH), 8.39 (s, 1H, N=CH), 7.70 (d, J = 8.67 Hz, 2H, Ar-H), 7.10 (d, J = 8.56 Hz, 2H, Ar-H), 6.77 (m, 4H, Ar-H), 3.00 (s, 6H, CH3). EMS: m/z: 241 [M + H]+.
Compound 8: 1H NMR (DMSO,500HZ) δ: 9.48 (s, 1H, OH), 8.38 (d, J = 8.78 Hz, 1H, N=CH), 7.63 (d, J = 7.39 Hz, 2H, Ar-H), 7.33-7.42 (3H, m, Ar-H), 7.26 (d, J = 15.96 Hz, 1H, Ar-H), 7.07-7.12 (m, 3H, Ar-H), 6.76 (d, 2H, J = 7.76 Hz, =CH). EMS: m/z: 224 [M + H]+.
Compound 9: 1H NMR (DMSO,500HZ) δ: 9.77 (s, 1H, OH), 8.39 (d, J = 8.78 Hz, 1H, N=CH), 7.77 (d, J = 7.39 Hz, 2H, Ar-H), 7.35–7.44 (3H, m, Ar-H), 7.28 (d, J = 15.96 Hz, 1H, Ar-H), 7.09–7.14 (m, 3H, Ar-H), 6.73 (d, 2H, J = 7.76 Hz, =CH). EMS: m/z: 224 [M + H]+.
Compound 10: 1H NMR (DMSO, 500 Hz) δ: 9.90 (s, 1H, OH), 8.41 (s, 1H, N=CH), 7.83 (d, J = 7.37 Hz, 1H), 7.20 (d, J = 8.73 Hz, 2H), 7.07 (t, J = 11.62 Hz, 1H), 6.80 (d, J = 8.32, 2H), 6.67 (d, J = 7.47 Hz, 1H), 6.53-6.46 (m, 1H). EMS: m/z:188 [M + H]+.
Rights and permissions
About this article
Cite this article
Zhang, Y., Zou, B., Wang, K. et al. Antioxidant activities and transition metal ion chelating studies of some hydroxyl Schiff base derivatives. Med Chem Res 21, 1341–1346 (2012). https://doi.org/10.1007/s00044-011-9648-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-011-9648-7