Abstract
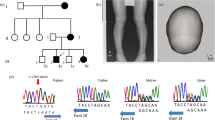
A 13-year-old Hungarian boy (B.J. Jr.) with congenital haemolytic anaemia (CHA) and hyperkinetic torsion dyskinesia was found to have severe triose-phosphate isomerase (TPI) deficiency. One of his two brothers (A.J.), a 23-year-old amateur wrestler, has CHA as well, but no neurological symptoms. Both have less than 10% TPI activity and a highly increased dihydroxyacetone phosphate (DHAP) level in their red blood cells. Their TPI had a slow electrophoretic mobility and was heat unstable. Both parents and a third brother are healthy heterozygous carriers of the defect. A.J. represents a unique phenotype from the point of view that all published “homozygotes” had severe neurological alterations from infancy or early childhood except one infant who died at 11 months, probably too young for neurological symptoms to be noted. In contrast to the two affected Hungarian brothers all but one “homozygote” has died before the age of 6 years. The striking difference in the clinical course of the defect between the two brothers with the same severe red blood cell enzyme deficiency may originate from unusual differences between two double heterozygous brothers resulting inter alia in different levels of TPI expression in various tissues. Significantly lower TPI activities were found in both the T- and B-cells of the propositus as compared to the respective cells of the neurologically symptom-free brother.
Similar content being viewed by others
References
Angelman H, Proin MC, MacIver IE (1970) A case of triosephosphate isomerase deficiency with sudden death (abstract). 13th International Congress of Hematology. Munich, p 122
Bardosi A, Eber SW, Hendrys M, Pekrum A (1990) Myopathy with altered mitochondria due to a triosephosphate isomerase (TPI) deficiency. Acta Neuropathol 79:387–394
Bellingham AJ, Williams LHP, Lestas AN, Nicolaides (1989) Prenatal diagnosis of a red cell enzymopathy: triosephosphate isomerase deficiency. Lancet II:419
Beutler E (1984) Red cell metabolism: a manual of biochemical methods. Grune & Stratton, Orlando, pp 11–12
Beutler E (1988) Selectivity of proteases as a basis for tissue distribution of enzymes in hereditary deficiencies. Proc Natl Acad Sci USA 80:3767–3768
Beutler E, Blume KG, Kaplan JC, Löhr GW, Ramot B, Valentine WN (1977) International Committee for Standardization in Haematology: recommended methods for red-cell enzyme analysis. Br J Haematol 35:331–340
Blacklow SC, Knowles JR (1990) How can a catalytic lesion be offset? The energetics of two pseudorevertant triosephosphate isomerases. Biochemistry 29:4099–4108
Blacklow SC, Liu KD, Knowles JR (1991) Stepwise improvements in catalytic effectiveness: independence and interdependence in combinations of point mutations of a sluggish triosephosphate isomerase. Biochemistry 30:8470–8476
Brown JR, Daar IO, Krug JR, Maquat LE (1985) Characterization of the functional gene and several processed pseudogenes in the human triosephosphate isomerase gene family. Mol Cell Biol 5:1694–1706
Clark ACL, Szobolotzky MA (1985) Triosephosphate isomerase deficiency: prenatal diagnosis. J Pediatr 106:417–420
Clay SA, Shore NA, Landing BH (1982) Triosephosphate isomerase deficiency. Am J Dis Child 136:800–802
Daar IO, Maquat LE (1988) Premature translation termination mediates triosephosphate isomerase mRNA degradation. Mol Cell Biol 8:802–813
Daar IO, Artymiuk PJ, Phillips DC, Maquat LE (1986) Human triosephosphate isomerase deficiency: a single amino acid substitution in a thermolabile enzyme. Proc Natl Acad Sci USA 83:7903–7907
Eber SW, Dünnwald M, Belohradsky BH, Bidlingmaier F, Schievelbein H, Weinmann HM, Krietsch WKG (1979) Hereditary deficiency of triosephosphate isomerase in four unrelated families. Eur J Clin Invest 9:195–202
Eber SW, Pekrum A, Bardosi A, Gahr M, Krietsch WKG, Krüger J, Matthei R, Schröter W (1991) Triosephosphate isomerase deficiency: haemolytic anaemia, myopathy with altered mitochondria and mental retardation due to a new variant with accelerated enzyme catabolism and diminished specific activity. Eur J Pediatr 150:761–766
Freycon F, Lauras B, Bovier-Lapierre F, Dorche CL, Goddon R (1975) Hereditary hemolytic anaemia with triosephosphate isomerase deficiency. Pediatrie 30:55–65
Hall JG (1990) Genomic imprinting: review and relevance to human diseases. Am J Hum Genet 46:857–873
Harris SR, Paglia DE, Jaffé ER, Valentine W, Klein RL (1978) Triosephosphate isomerase deficiency in an adult. Clin Res 18:529–535
Hollán S, Miwa S, Fujii H, Natonek K, Hirono A, Hirono K, Kannom H (1990) An unique experiment of nature: a 21-year-old symptom free homozygote for triosephosphate isomerase (TPI) deficiency (abstract). Blood 76 [Suppl]
Jongsma AP, Los WR, Hagemeier A (1974) Proceedings: evidence for synteny between the human loci for triosephosphate isomerase lactate dehydrogenase-B, and peptidase-B and the regional mapping of these loci on chromosome 12. Cytogenet Cell Genet 13:106–107
Joseph D, Petsko A, Karplus M (1990) Anatomy of a conformational change: hinged lid motion of the triosephosphate isomerase loop. Science 249:1425–1428
Knowles JR (1991) Enzyme catalysis: not different, just better. Nature 350:121–124
Komives EA, Chang LC, Lolis E, Tilton RF, Petsko GA, Knowles JR (1991) Electrophilic catalysis in triosephosphate isomerase: the role of histidin 95. Biochemistry 30:3011–3019
Kucherlapati RS, Creagan RP, Nichols EA, Borgaoncar DS, Ruddle FH (1975) Synteny relationships of four human genes: mannose phosphate isomerase to pyruvate kinase-3 and triosephosphate isomerase to lactate dehydrogenase-B. Cytogenet Cell Genet 14:364–367
Lodi PJ, Knowles JR (1991) Neutral imidazole is the electrophil in the reaction catalyzed by triosephosphate isomerase: structural origins and catalytic implications. Biochemistry 30:6948–6956
Lolis E, Petsko GA (1990) Crystallographic analysis of the complex between triosephosphate isomerase and 2-phosphoglycolate at 2. 5-Å resolution: implications for catalysis. Biochemistry 29:6619–6625
Maquat LE, Chilcote R, Ryan PM (1985) Human triosephosphate isomerase cDNA and protein structure. J Biol Chem 260:3748–3753
Merkle S, Pretsch W (1989) Characterization of triosephosphate isomerase with reduced enzyme activity in Mus musculus. Genetics 123:837–844
Minakami S, Suzuki C, Caito T, Yoshikawa H (1965) Studies on erythrocyte glycolysis. I. Determination of the glycolytic intermediates in human erythrocytes. J Biochem 58:543–550
Miwa S, Luzzatto L, Rosa R, Paglia DE (1989) Schröter M, De Flora A, Fujii H, Board PG, Beutler E (1989) International Committee for Standardization in Haematology: recommended ethods for an additional red cell enzyme (pyrimidine 5-nucleotidase) assay and determination of red cell adenosine 5triphosphate, 2,3-diphosphoglycerate and reduced glutathione. Clin Lab Haematol 11:131–138
Mohrenweiser HW, Fielek S (1982) Elevated frequency of carriers for triosephosphate isomerase deficiency in newborn infants. Pediatr Res 16:960–963
Nakashima K, Ogawa H, Oda S, Miwa S (1973) 1,3-Diphosphoglycerate in phosphoglycerate kinase deficiency. Clin Chim Acta 49:455–458
O'Dorisio MS, Panerai A (eds) (1990) Neuropeptides and Immunopeptides: messengers in a neuroimmune axis. Ann NY Acad Sci 594:1–501
Poll-The BT, Aicardi J, Girot R, Rosa R (1985) Neurological findings in triosephosphate isomerase deficiency. Ann Neurol 17:439–443
Pompliano DL, Peyman A, Knowles JR (1990) Stabilization of a reaction intermediate: definition of the functional role of the flexible loop in triosephosphate isomerase. Biochemistry 29:3186–3194
Rosa R, Prehu MO, Beuzard Y, Rosa J (1978) The first case of a complete deficiency of diphospholycerate mutase in human erythrocytes. Clin Invest 62:907–915
Rosa R, Prehu M-O, Calvin M-C, Badonal J, Alix D, Girod R (1985) Hereditary triose phosphate isomerase deficiency: seven new homozygous cases. Hum Genet 71:235–240
Rose IA, Fung WJ, Warm JVB (1990) Proton diffusion in the active site of triosephosphate isomerase. Biochemistry 29:4312–4317
Sampson NS, Knowles JR (1992a) Segmental movement: definition of the structural requirements for loop closure in catalysis by triosephosphate isomerase. Biochemistry 31:8482–8487
Sampson NS, Knowles JR (1992b) Segmentai motion in catalysis: investigation of a hydrogen bond critical for loop closure in the reaction of triosephosphate isomerase. Biochemistry 31:8488–8494
Satoh Ch, Neel JV, Yamashita A, Goriki K, Fujita M, Hamilton HB (1983) The frequency among Japanese of heterozygotes for deficiency variants of 11 enzymes. Am J Hum Genet 35:656–674
Schneider AS, Valentine WN, Hattori M, Heins Jr HL (1965) Hereditary hemolytic anaemia with triosephosphate isomerase deficiency. N Engl J Med 272:229–235
Skala H, Dreyfus JC, Vives-Corrons JL, Matsumoto F, Beutler E (1977) Triosephosphate isomerase deficiency. Biochem Med 18:226–234
Szelényi J, Páldi-Haris P, Hollán S (1987) Changes in the cholinergic system of lymphocytes due to mitogenic stimulation. Immunol Lett 16:49–54
Valentine WN, Schneider AS, Baughan MA, Paglia DE, Heins Jr HL (1966) Hereditary hemolytic anaemia with triosephosphate isomerase deficiency. Am J Med 41:27–41
Vives-Corrons JL, Rubinson-Skala H, Mateo M, Estella J, Felin E, Dreyfus JC (1978) Triosephosphate isomerase deficiency with hemolytic anaemia and severe neuromuscular disease: familial and biochemical studies of a case found in Spain. Hum Genet 42:171–180
Werner C, Klouda PT, Correa MC, Vassali P, Jeannet M (1977) Isolation of B and T lymphocytes by nylon fiber columns. Tissue Antigens 9:227–229
Zanella A, Mariani M, Colombo MB, Borgna-Pignatti C, De Stefano P, Morgese G, Sirchia G (1985) Triosephosphate isomerase deficiency: 2 new cases. Scand J Haematol 34:417–424
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hollán, S., Fujii, H., Hirono, A. et al. Hereditary triosephosphate isomerase (TPI) deficiency: two severely affected brothers one with and one without neurological symptoms. Hum Genet 92, 486–490 (1993). https://doi.org/10.1007/BF00216456
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00216456