Abstract
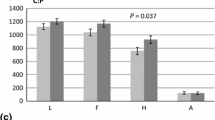
Elevated concentrations of O3 and CO2 have both been shown to affect structure, nutrient status, and deposition of secondary metabolites in leaves of forest trees. While such studies have produced robust models of the effects of such air pollutants on tree ecophysiology and growth, few have considered the potential for broader, ecosystem-level effects after these chemically and structurally altered leaves fall as leaf litter and decay. To determine the effects of elevated O3 and/or CO2 on the subsequent decomposition and nutrient release from the leaves grown in such altered atmospheres, we grew seedlings of three widespread North American forest trees, black cherry (Prunus serotina) (BC), sugar maple (Acer saccharum) (SM), and yellow-poplar (Liriodendron tulipifera) (YP) for two growing seasons in charcoal-filtered air (CF-air=approximately 25% ambient O3), ambient O3 (1X) or twice-ambient O3 (2X) in outdoor open-top chambers. We then assayed the loss of mass and N from the litter derived from those seedlings through one year litterbag incubations in the forest floor of a neighboring forest stand. Mass loss followed linear functions and was not affected by the O3 regime in which the leaves were grown. Instantaneous decay rates (i.e. k values) averaged SM:−0.707 y-1, BC:−0.613 y-1, and YP:−0.859 y-1. N loss from ambient (1X) O3-grown SM leaves was significantly greater than from CF-air leaves: N loss from BC leaves did not differ among treatments. Significantly less N was released from CF-air-grown YP leaves than from 1X or 2X O3-treated leaves. YP leaves from plants grown in pots at 2X O3 and 350 ppm supplemental CO2 in indoor pollutant fumigation chambers (CSTRs or Continuously Stirred Tank Reactors) loss 40% as much mass and 27% as much N over one year as did leaves from YP grown in CF-air or 2X O3. Thus, for leaves from plants grown in pots in controlled environment fumigation chambers, the concentrations of both O3 and CO2 can affect N release from litter incubated in the field whereas mass loss rate was affected only by CO2. Because both mass loss and N release from leaves grown at elevated CO2 were reduced significantly (at least for yellow-poplar), forests exposed to elevated CO2 may have significantly reduced N turnover rates, thereby resulting in increased N limitation of tree growth, especially in forests which are already N-limited.
Similar content being viewed by others
References
Brown K R 1991 Carbon dioxide enrichment accelerates the decline in nutrient status and relative growth rate ofPopulus tremuloides Michx. seedlings. Tree Physiol. 8, 161–173.
Buecker J and Ballach H-J 1992 Alterations in carbohydrate levels in leaves ofPopulus due to ambient air pollution. Physiol. Plant. 86, 512–517.
Chappelka A and Chevone B I 1988 Growth and physiological responses of yellow-poplar seedlings exposed to ozone and simulated acid rain. Environ. Pollut. 49, 1–18.
Couteaux M-M, Mousseau M, Celerier M-L and Bottner P 1991 Increased atmospheric CO2 and litter quality: decomposition of sweet chestnut leaf litter with animal food webs of different complexities. Oikos 61, 54–64.
Davis D D and Skelly J M 1992a Foliar sensitivity of eight eastern hardwood species to ozone. Water Air Soil Pollut 62, 269–277.
Davis D D and Skelly J M 1992b Growth response of four species of eastern hardwood tree seedlings exposed to ozone, acidic precipitation, and sulfur dioxide. J. Air Pollut. Control Assoc. 42, 309–311.
Findlay S and Jones G C 1990 Exposure of cottonwood plants to ozone alters subsequent leaf decomposition. Oecologia 82, 248–250.
Guderian R, Tingey D T and Rabe R 1985 Effects of photochemical ozidants on plants.In Air Pollution by Photochemical Oxidants. Ed. R Guderian. pp 129–146. Springer-Verlag, NY.
Hassig B E and Dickson R E 1979 Starch measurement in plant tissue using enzymatic hydrolysis. Plant Physiol. 47, 151–157.
Heagle A S, Body D E and Heck W W 1973 An open-top field chamber to assess the impact of air pollution on plants. J. Environ. Qual. 2, 365–368.
Heck W W, Philbeck R B and Dunning J A 1978 A continuously stirred tank reactor (CSTR) system for exposing plants to gaseous air contaminants. U.S.D.A. Agricultural Research Service Report ARS-S-181. Washington, DC.
Hill A C and Littlefield N 1969 Ozone: Effect on apparent photosynthesis, rate of transpiration, and stomatal closure in plants. Environ. Sci. Technol. 3, 52–56.
Jensen K F 1973 Response of nine forest tree species to chronic ozone fumigation. Plant Dis. Rep. 57, 914–917.
Keen T and Taylor O C 1975 Ozone injury in soybeans: isoflavonoid accumulation is related to necrosis. Plant Physiol. 55, 731–733.
Lambers H 1993 Rising CO2, secondary plant metabolism, plant-herbivore interactions and litter decomposition. Vegetatio 104/105, 263–271.
Lindroth R L, Kinney K L and Platz C L 1993 Responses of deciduous trees to elevated atmospheric CO2: Productivity, phytochemistry, and insect performance. Ecology 74, 763–777.
Manion P D and Lachance D 1992 Forest Decline Concepts. APS Press, St. Paul, MN.
McConnaughay K D M, Bernston G M and Bazzaz F A 1993 Plant responses to carbon dioxide. Nature 361, 24.
Noble R D and Jensen K F 1980 Effects of sulfur dioxide and ozone on growth of hybrid poplar leaves. Am. J. Bot. 67, 1005–1009.
Olson J S 1963 Energy storage and the balance of producers and decomposers in ecological systems. Ecology 43, 322–331.
Pell E J and Pearson N S 1984 Ozone-induced reduction in quantity and quality of two potato cultivars. Environ Pollut. 35, 345–352.
Pye J M 1988 Impact of ozone on the growth and yield of trees: a review. J. Environ. Qual. 17, 347–360.
Reich P B and Amundson R G 1985 Ambient levels of ozone reduce net photosynthesis in tree and crop species. Science 230, 566–570.
Reich P B and Lassoie J P 1985 Influence of low concentrations of ozone on growth, biomass partitioning, and leaf senescence in young hybrid poplar plants. Environ. Pollut. 39, 39–51.
Reich P B and Tomlinson H 1974 Mechanisms of ozone injury to plants.In Air Pollution Effects on Plant Growth. Ed. M Dugger. pp 76–81. American Chemical Society, Washington, DC.
Reich P B, Schoettle A W and Amundson R G 1986 Effects of O3 and acid rain on photosynthesis and growth in sugar maple and northern red oak seedlings. Environ. Pollut. 40, 1–15.
S A S 1985 Statistical Analysis System User's Guide: Statistics. S.A.S. Institute, Cary NC.
Scherzer A J and Boerner R E J 1994 Effects of two years of nearambient ozone on sugar maple (Acer saccharum) seedlings. I. Growth, carbohydrate storage and phenotypic variation. Environ. Exp. Bot. (In press).
Scherzer A J and McClenahen J R 1989 Effects of ozone or sulfur dioxide on pitch pine seedlings. J. Environ. Qual. 18, 57–61.
Technicon 1977 Individual/simultaneous determination of nitrogen and/or phosphorus in BD acid digests: Industrial methods 32974W/B. Technicon Industrial Systems, Tarrytown, NY.
Van Soest P J 1963 Use of detergents in the analysis of fibrous feeds. II. A rapid method for the determination of fiber and lignin. J Assoc. Off Anal. Chem 49, 546–551.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Boerner, R.E.J., Rebbeck, J. Decomposition and nitrogen release from leaves of three hardwood species grown under elevated O3 and/or CO2 . Plant Soil 170, 149–157 (1995). https://doi.org/10.1007/BF02183063
Issue Date:
DOI: https://doi.org/10.1007/BF02183063