Abstract
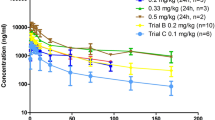
A Phase I photodynamic therapy (PDT) clinical trial was carried out with Temoporfin (Foscan®, mTHPC) at the Departments of Otolaryngology at Orebro Medical Center (OMC) and Long Island Jewish Medical Center (LIJMC). A range of drug doses, consisting of 0.3, 0.15, 0.075 and 0.0375 mg kg−1, were utilized. Light treatment was performed on the sixth day after injection of the photosensitizer mTHPC. Photodynamic therapy was done on prostate cancer (six cases), bronchial cancer (one case), nasopharyngeal cancer (three cases), laryngeal cancer (eight cases), mesothelioma (one case), laryngeal papilloma (five cases) and basal cell nevus syndrome (one case). A number of patients were treated more than once. Plasma was collected and analysed at 1, 24, 48, 72, 96, 120 and 144 h and at 2 weeks post-injection, to follow the loading and clearance rate of the photosensitizer. Normal and malignant tissues were collected immediately prior to PDT, chemically extracted, and analysed for drug content spectrofluorometrically. Plasma drug levels were proportional to the dose. The half-life of the drug was 45.4 h across the entire dose range. The ratio of the drug in the tumour compared to normal adjacent mucosa was in the range of 2–3. There were no significant adverse effects. These data establish the basis for full clinical trials.
Similar content being viewed by others
References
von Tappiener SH, Jesionek A. Therapeutische versiche mit fluoreszierenden Stoffen.Muench Med Wochenschr 1903,50:2042–4
Dougherty TJ, Kaufman JE, Goldfarb A, Weishaupt KR, Boyle D, Mittleman A. Photoradiation therapy for the treatment of malignant tumours.Cancer Res 1978,38:2628–35
Bonnett R. Photosensitizers of the porphyrin and phthalocyanine series for photodynamic therapy.Chem Soc Rev 1995,24:19–33
Proceedings, VI Biennial Meeting of the International Photodynamic Association, Melbourne, March 1996
Lofgren LA, Ronn AM, Abramson AL et al. Photodynamic therapy using meso-tetra (hydroxyphenyl) chlorin: an animal model.Arch Otol Head Neck Surg 1994,120:1355–62
Lofgren LA, Ronn AM, Abramson AL, Shikowitz MJ, Nouri M, Steinberg BM. A standardized methodology for evaluation of photoactive anti-tumor agents as applied to meta-tetra (hydroxyphenyl) chlorin. In: Horrobin DF (ed)New Approaches to Cancer Treatment. London: Churchill Communications Europe, 1994:133–41
Abramson AL, Lofgren LA, Ronn AM, Nouri M, Steinberg BM. Treatment effects of meta-tetra (hydroxyphenyl) chlorin on the larynx. In: Horrobin DF (ed)New Approaches to Cancer Treatment. London: Churchill Communications Europe, 1994:142–7
Berenbaum MC. Comparison of hematoporphyrin derivatives and new photosensitizers. In:Photosensitizing Compounds: Their Chemistry, Biology and Clinical Use, Ciba Foundation Symposium, Vol. 146. Chichester: John Wiley and Sons Inc., 1989:33–40
Bonnett R, White RD, Winfield UJ, Berenbaum MC. Hydroporphyrins of the meso-tetra (hydroxyphenyl) porphyrin series as tumor photosensitizers.Biochem J 1989, 260:277–80
Lofgren LA, Ronn AM. Meso-tetra (hydroxyphenyl) chlorin—An in vivo photodegradation study.Proceedings of Optical Methods for Tumor Treatment and Detection: Mechanisms and Techniques in Photodynamic Therapy III 1994,2133:112–5
Ronn AM, Batti J, Lee CJ et al. Comparative biodistribution of m-THPC in multiple species: clinical implications for photodynamic therapy.Lasers Surg Med (in press)
Lofgren LA, Ronn AM. Tissue dependent biosynthesis and pharmacokinetics of Protoporphyrin IX following intravenous injection of aminolevulinic acid.Proceedings of Optical Methods for Tumor Treatment and Detection: Mechanisms and Techniques in Photodynamic Therapy IV 1995,2392:85–92
Peng Q, Moan J, Ma L-W, Nesland JM. Uptake, localization, and photodynamic effect of meso-Tetra(hydroxyphenyl)porphine and its corresponding chlorin in normal and tumor tissues of mice bearing mammary carcinoma.Cancer Res 1995,55:2620–6
Fisher AMR, Murphree AL, Gomer CJ. Clinical and preclinical photodynamic therapy.Lasers Surg Med 1995,17:2–31
Korbelik M, Krosl G. Cellular levels of photosensitizers in tumors: the role of proximity to the blood supply.Breast Cancer 1994,70:604–10
Ma L, Moan J, Berg K. Evaluation of a new photosensitizer, meso-tetra-hydroxyphenyl-chlorin, for use in photodynamic therapy: a comparison of its photobiological properties with those of two other photosensitizers.Int J Cancer 1994,57:883–888
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ronn, A.M., Nouri, M., Lofgren, L.A. et al. Human tissue levels and plasma pharmacokinetics of temoporfin (Foscan®, mTHPC). Laser Med Sci 11, 267–272 (1996). https://doi.org/10.1007/BF02134918
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02134918