Abstract
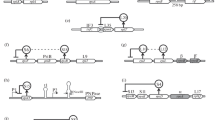
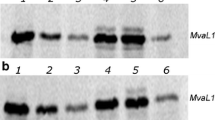
The work carried out in the authors' laboratories on the structure and expression of ribosomal protein genes inXenopus is reviewed, with some comparisons with other systems. These genes form a class that shares several structural features, especially in the region surrounding the 5′ ends. These similar structures appear to be involved in coregulated expression that is attained at various regulatory levels: transcriptional, transcript processing and stability, and translational. Particular attention is paid here to the one operating at the translational level, which has been studied duringXenopus oogenesis and embryogenesis, and also during nutritional changes ofXenopus cultured cells. This regulation, which responds to the cellular need for new ribosomes, operates by changing the fraction of rp-mRNA engaged on polysomes, leaving each translated rp-mRNA molecule always fully loaded with ribosomes. Responsible for this translational behaviour is the typical 5′UTR, which characterizes all rp-mRNAs analyzed up to now, and that can bindin vitro some proteins, putative trans-acting factors for this translational regulation.
Similar content being viewed by others
References
Amaldi, F., I. Bozzoni, E. Beccari & P. Pierandrei-Amaldi, 1989. Expression of ribosomal protein genes and regulation of ribosome biosynthesis inXenopus development. Trends Biochem. Sci. 14: 175–178.
Amaldi, F. & Pierandrei-Amaldi, 1990. Translational regulation of the expression of ribosomal protein genes inXenopus laevis. Enzyme 44: 93–105.
Bagni, C., P. Mariottini, F. Annesi & F. Amaldi, 1990. Structure ofXenopus laevis ribosomal protein L32 and its expression during development. Nucl. Acids Res. 18: 4423–4426.
Bagni, C., P. Mariottini, L. Terrenato & F. Amaldi, 1992. Individual variability in the translational regulation of ribosomal protein synthesis inXenopus. Mol. Gen. Genet. 234: 60–64.
Baum, E.Z., L.E. Hyman & W.M. Wormington, 1988. Post-translational control of ribosomal protein L1 accumulation inXenopus oocytes. Dev. Biol. 126: 141–146.
Baum, E.Z. & W.M. Wormington, 1985. Cooroinate expression of r-protein genes duringXenopus development. Dev. Biol. 1111: 488–498.
Beccari, E. & P. Mazzetti, 1987. The nucleotide sequence of the ribosomal protein L14 gene ofXenopus laevis. Nucl. Acids Res. 15: 1870–1872.
Beccari, E., P. Mazzetti, A.M. Mileo, I. Bozzoni, P. Pierandrei-Amaldi & F. Amaldi, 1986. Sequences coding for the ribosomal protein L14 inXenopus laevis andXenopus tropicalis: homologies in the 5′ non translated region are shared with other r-protein mRNAs. Nucl. Acids Res. 14: 7633–7646.
Bisbee, C.A., M.A. Baker, A.C. Wilson, I. Hadji-Azimi & M. Fishberg, 1977. Albumin phylogeny for clawed frogs (Xenopus). Science 195: 785–787.
Bowman, L.H., 1987. The synthesis of ribosomal proteins S16 and L32 is not autogenously regulated during mouse myoblast differentiation. Mol Cell Biol 7: 4464–4471.
Bozzoni, I., P. Fragapane, F. Annesi, P. Pierandrei-Amaldi, F. Amaldi & E. Beccari, 1984. Expression of twoXenopus laevis ribosomal protein genes in injected frog oocytes. A specific splicing block interferes with the L1 RNA maturation. J. Mol. Biol. 180: 987–1005.
Brown, D.D. & I.B. Dawid, 1968. Specific gene amplification in oocytes. Science 160: 272–280.
Brown, D.D. & J.B. Gurdon, 1964. Absence of rRNA synthesis in the anucleolate mutant ofXenopus laevis. Proc. Natl. Acad. Sci. USA 51: 139–146.
Caffarelli, E., P. Fragapane, C. Gehring & I. Bozzoni, 1987. The accumulation of mature RNA for theXenopus laevis ribosomal protein L1 is controlled at the level of splicing and turnover of the precursor RNA. EMBO J. 6: 3493–3498.
Caizergues-Ferrer, M., P. Mariottini, C. Curie, B. Lapeyre, N. Gas, F. Amalric & F. Amaldi, 1989. Nucleolin fromXenopus laevis: cDNA cloning and expression during development. Genes Dev. 3: 324–333.
Cardinali, B., N. Campioni & P. Pierandrei-Amaldi, 1987. Ribosomal protein, histone and calmodulin mRNAs are differently regulated at the translational level during oogenesis ofXenopus laevis. Exp. Cell Res. 169: 432–441.
Cardinali, B., M. Di Cristina & P. Pierandrei-Amaldi, 1993. Interaction of proteins with the mRNA for ribosomal protein L1 inXenopus: structural characterization ofin vivo complexes and identification of proteins that bindin vitro to its 5′UTR. Nucl. Acids Res. 21: 2301–2308.
Carnevali, F., C. La Porta, V. Ilardi & E. Beccari, 1989. Nuclear factors specifically bind to upstream sequences of aXenopus laevis ribosomal protein gene promoter. Nucl. Acids Res. 17: 8171–8184.
Cecconi, F., P. Mariottini, F. Loreni, P. Pierandrei-Amaldi, N. Campioni & F. Amaldi, 1994. U17XS8, a small nucleolar RNA with a 12 nt complementarity to 18S rRNA and coded by a sequence repeated in the six introns ofXenopus laevis ribosomal protein S8 gene. Nucl. Acids Res. in press.
Chen, Q.-M., P. Mariottini, C. Bagni & F. Amaldi, 1992. The pyrimidine sequence encompassing the transcription start point ofXenopus laevis ribosomal-protein-encoding genes it not obligatory for activity in oocytes. Gene 119: 283–286.
Cutruzzolà, F., F. Loreni & I. Bozzoni, 1986. Complementarity of conserved sequence elements present in 28S ribosomal RNA and in ribosomal protein genes ofXenopus laevis andXenopus tropicalis. Gene 49: 371–376.
Davidson, E.H., 1986. Gene activity in early development, Academic Press, Orlando.
Di Cristina, M., R. Menard & P. Pierandrei-Amaldi, 1991.Xenopus laevis ribosomal protein Sla cDNA sequence. Nucl. Acids Res. 19: 1943.
Elsdale, T.R., M. Fischberg & S. Smith, 1958. A mutation that reduces nucleolar number inXenopus laevis. Exp. Cell Res. 14: 642–643.
Fragapane, P., E. Caffarelli, M. Lener, S. Prislei, B. Santoro & I. Bozzoni, 1992. Identification of the sequences responsible for the splicing phenotype of the regulatory intron of the L1 ribosomal protein gene ofXenopus laevis. Mol. Cell. Biol. 12: 1117–1125.
Fragapane, P., S. Prislei, A. Michienzi, E. Caffarelli & I. Bozzoni, 1993. A novel small nucleolar RNA (U16) is encoded inside a ribosomal protein intron and originates by processing of the pre-mRNA. EMBO J. 12: 2921–2928.
Gall, J.G., 1968. Differential synthesis of the genes for ribosomal RNA during amphibian oogenesis. Proc. Natl. Acad. Sci. USA 60: 553–560.
Geyer, P.K., O. Meyuhas, R.P. Perry & L.F. Johnson, 1982. Regulation of ribosomal protein mRNA content and translation in growth-stimulated mouse fibroblasts. Mol. Cell. Biol. 2: 685–693.
Hammond, M.L., W. Merrick & L.H. Bowman, 1991. Sequences mediating the translation of mouse S16 ribosomal protein mRNA during myoblast differentiation andin vitro and possible control points for thein vitro translation. Genes Dev. 5: 1723–1736.
Hariharan, M., D. Kelley & R.P. Perry, 1989. Equipotent mouse ribosomal protein promoters have a similar architecture that includes internal sequence elements. Genes Dev. 3: 1789–1800.
Hariharan, M.D. & R.P. Perry, 1990. Functional dissection of a mouse ribosomal protein promoter: significance of the polypyrimidine initiator and an element in the TATA-box region. Proc. Natl. Acad. Sci. USA 87: 1526–1530.
Hyman, L.E. & W.M. Wormington, 1988. Translational inactivation of ribosomal protein mRNAs duringXenopus oocyte maturation. Genes Dev. 2: 598–605.
Kaspar, R.L., K. Tomohito, H. Cranston, D.R. Morris & M.W. White, 1992. A regulatory cis element and a specific binding factor involved in the mitogenic control of murine ribosomal protein L32 translation. J. Biol. Chem. 267: 508–514.
Kiss, T. & W. Filipowicz, 1993. Small nucleolar RNAs encoded by introns of the human cell cycle regulatory gene RCCI. EMBO J. 12: 2913–2920.
Leverette, R.D., M.T. Andrews & E.S. Maxwell, 1992. Mouse U14 snRNA is a processed intron of the cognate hsc70 heat shock pre-messenger RNA. Cell 71: 1215–1221.
Levy, S., D. Avni, M. Hariharan, R.P. Perry & O. Meyuhas, 1991. Oligopyrimidine tract at the 5′ end of mammalian ribosomal protein mRNAs is required for their translational control. Proc. Natl. Acad. Sci. USA 88: 3319–3323.
Liu, J. & E.S. Maxwell, 1990. Mouse U14 snRNA is encoded in an intron of the mouse cognate hsc70 heat shock gene. Nucl. Acids Res. 18: 6565–6571.
Lodish, H.F., 1974. Model for the regulation of mRNA translation applied to haemoglobin synthesis. Nature 251: 385–388.
Loreni, F. & F. Amaldi, 1992. Translational regulation of ribosomal protein synthesis inXenopus cultured cells: mRNA relocation between polysomes and RNP during nutritional shifts. Eur. J. Biochem. 105: 1027–1032.
Loreni, F., A. Francesconi & F. Amaldi, 1993. Coordinate translational regulation in the syntheses of elongation factor 1α and ribosomal proteins inXenopus laevis. Nucl. Acids Res. 21: 4721–4725.
Loreni, F., A Francesconi, R. Jappelli & F. Amaldi, 1992. Analysis of mRNAs under translational control duringXenopus embryogenesis: isolation of new ribosomal protein clones. Nucl. Acids Res. 20: 1859–1863.
Loreni, F., I. Ruberti, I. Bozzoni, P. Pierandrei-Amaldi & F. Amaldi, 1985. Nucleotide sequence of the L1 ribosomal protein gene ofXenopus laevis: remarkable sequence homology among introns. EMBO J. 4: 3483–3488.
Mager, W.H., 1988. Review: Control of ribosomal protein gene expression. Biochim. Biophys. Acta 949: 1–15.
Marchioni, M., S. Morabito, A.L. Salvati, E. Beccari & F. Carnevali, 1993. XrpFl, an amphibian transcription factor composed of multiple polypeptides immunologically related to the GA binding protein α and β subunits, is differentially expressed duringXenopus laevis development. Mol. Cell. Biol. 13: 6479–6489.
Mariottini, P., F. Amaldi, 1990. The 5′ untranslated region of mRNA for ribosomal protein S19 is involved in its translation duringXenopus development. Mol. Cell. Biol. 10: 816–822.
Mariottini, P., C. Bagni, F. Annesi & F. Amaldi, 1988. Isolation and nucleotide sequences of cDNAs forXenopus laevis ribosomal protein S8: similarities in the 5′ and 3′ untranslated regions of mRNAs for different r-proteins. Gene 67: 69–74.
Mariottini, P., C. Bagni, A. Francesconi, F. Cecconi, M.J. Serra, Q.-M. Chen, F. Loreni, F. Annesi & F. Amaldi, 1993. Sequence of the gene coding for ribosomal protein S8 ofXenopus laevis. Gene 132: 255–260.
Meyuhas, O., E.A. Thompson & R.P. Perry, 1987. Glucocorticoid selectively inhibit translation of ribosomal protein mRNAs in P1798 lymphosarcoma cells. Mol. Cell. Biol. 7: 2691–2699.
Nomura, M., 1990. History of ribosome research: a personal account, pp. 3–55 in The Ribosome: Structure, Function and Evolution, edited by W. Hill et al., Amer. Soc. Microbiol, Washington.
Perry, R.P. & O. Meyuhas, 1990. Translational control of ribosomal protein production in mammalian cells. Enzyme 44: 83–92.
Pierandrei-Amaldi, P., F. Amaldi, I. Bozzoni & P. Fragapane, 1987. Regulation of ribosomal protein genes duringXenopus development, pp 267–278 in Molecular Approaches to Developmental Biology: UCLA Symposia on Molecular and Cellular Biology. vol 51, edited by R.A. Firtel and E.H. Davidson, Alan R. Liss, New York.
Pierandrei-Amaldi, P. & E. Beccari, 1981. Messenger RNA for ribosomal proteins inXenopus laevis oocytes. Eur. J. Biochem. 106: 603–611.
Pierandrei-Amaldi, P., E. Beccari, I. Bozzoni & F. Amaldi, 1985a. Ribosomal protein production in normal and anucleolateXenopus embryos: regulation at the posttranscriptional and translational levels. Cell 42: 317–323.
Pierandrei-Amaldi, P., I. Bozzoni & B. Cardinali. 1988. Expression of the gene for ribosomal protein L1 inXenopus embryos: alteration of gene dosage by microinjection. Genes Dev. 2: 23–31.
Pierandrei-Amaldi, P., N. Campioni, E. Beccari, I. Bozzni & F. Amaldi, 1982. Expression of ribosomal protein genes inXenopus laevis development. Cell 30: 163–171.
Pierandrei-Amaldi, P., N. Campioni & B. Cardinali, 1991. Experimental changes in the amount of maternally stored ribosomes affect the translation efficiency of ribosomal protein mRNA inXenopus embryo. Cell. Mol. Biol. 37: 227–238.
Pierandrei-Amaldi, P., N. Campioni, P. Gallinari, E. Beccari, I. Bozzoni & F. Amaldi, 1985b. Ribosomal protein synthesis is not autogenously regulated at the translational level inXenopus laevis. Dev. Biol. 167: 281–289.
Planta, R.J., W.H. Mager, R.J. Leer, L.P. Wouldt, H.A. Raué & T.T.A.L. El-Baradi, 1986. Structure and expression of ribosomal protein genes in yeast, pp. 699–718 in Structure, Function and Genetics of Ribosomes, edited by B. Hardesty and G. Kramer. Springer Verlag, New York.
Planta, R.J. & H.A. Raué, 1988. Control of ribosome biogenesis in yeat. Trends Genet. 4: 64–68.
Prislei, S., A. Michienzi, C. Presutti, P. Fragapane & I. Bozzoni, 1993. Two different snoRNAs are encoded in introns of amphibian and human L1 ribosomal protein genes. Nucl. Acids Res. 21: 5824–5830.
Ray, B.K., T.G. Brendler, S. Adya, S. Daniels-McQueen, J.K. Miller, J.W.B. Hershey, J.A. Grifo, W.C. Merrick & R.E. Thach, 1983. Role of mRNA competition in regulatory translation: Further characterization of mRNA discriminatory initiation factors. Proc. Natl. Acad. Sci. USA 80: 663–667.
Steel, L.F. & A. Jacobson, 1991: Sequence elements that affect mRNA translation activity in developingDictyostelium cells. Dev. Genetics 12: 98–103.
Tycowski, K.T., M.-D. Shu & J.A. Steitz, 1993. A small nucleolar RNA is processed from an intron of the human gene encoding ribosomal protein S3. Genes & Dev 7: 1176–1190.
Wagner, M. & R.P. Perry, 1985. Characterization of the multigene family encoding the mouse S16 ribosomal protein: strategy for distinguishing an expressed gene from its processed pseudogene counterparts by an analysis of total genomic DNA. Mol. Cell. Biol. 5: 3560–3576.
Wallace, H.R. & M.L. Birnstiel, 1966. Ribosomal cistrons and the nucleolar organizer. Biochim. Biophys. Acta 114: 296–310.
Warner, J.R., D.M. Baronas-Lowell, F.J. Eng, S.P. Johnson, Q. Ju & B.E. Morrow, 1990. Genetic approaches to ribosome biosynthesis in the yeastSaccharomyces cerevisiae, pp. 699–718 in The Ribosome: Structure, Function and Evolution, edited by W.E. Hill, A. Dahlberg, R.A. Garrett, P.B. Moore, D. Schlessinger & J.R. Warner. American Society for microbiology, Washington, D.C.
Weiss, Y.C., C.A. Vaslet & M. Rosbash, 1981. Ribosomal protein mRNAs increase dramatically duringXenopus development. Dev. Biol. 87: 330–339.
White, M.W., C. Degnin, J. Hill & D.R. Morris, 1990. Specific regulation by endogenous polyamines of translational initiation of S-adenosylmethionine decarboxylase mRNA in swiss 3T3 fibroblasts. Biochem. J. 268: 657–660.
Wormington, W.M., 1986. Stable repression of ribosomal protein L1 synthesis inXenopus oocytes by microinjection of antisense RNA. Proc. Natl. Acad. Sci. USA 83: 8639–8643.
Wormington, M.W., 1989. Developmental expression and 5S binding activity ofXenopus ribosomal protein L5. Mol. Cell. Biol. 9: 5281–5288.
Wormington, W.M., Schlissel & D.D. Brown, 1983. Developmental regulation ofXenopus 5S RNA genes. Cold Spring Harbor Symp. Quant. Biol. 47: 879–884.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Pierandrei-Amaldi, P., Amaldi, F. Aspects of regulation of ribosomal protein synthesis inXenopus laevis . Genetica 94, 181–193 (1994). https://doi.org/10.1007/BF01443432
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF01443432