Abstract
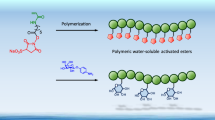
Several types of polymeric glycoconjugates,N-substituted polyacrylamides, have been synthesized by the reaction of activated polymers with ω-aminoalkylglycosides: (i) (carbohydrate-spacer) n -polyacrylamide, ‘pseudopolysaccharides’; (ii) (carbohydrate-spacer) n -phosphatidylethanolamine m -polyacrylamide, neoglycolipids, derivatives of phosphatidylethanolamine; (iii) (carbohydrate-spacer) n -biotin m -polyacrylamide, biotinylated probes; (iv) (carbohydrate-spacer) n -polyacrylamide-(macroporous glass), affinity sorbents based on macroporous glass, covalently coated with polyacrylamide. An almost quantitative yield in the conjugation reaction makes it possible to insert in the conjugate a predetermined quantity of the ligand(s).
Pseudopolysaccharides proved to be a suitable form of antigen for activation of polystyrene and poly(vinyl chloride) plates (ELISA) and nitrocellulose membranes (dot blot), being advantageous over traditional neoglycoproteins. Polyvalent glycolipids insert well in biological membranes: their physical properties, particularly solubility, can be changed in a desired direction. Biotinylated derivatives were used as probes for detection and analysis of lectins.
Similar content being viewed by others
Abbreviations
- sp:
-
spacer arm
- PAA:
-
polyacrylamide
- PE:
-
phosphatidylethanolamine
- Biot:
-
biotin
- MPGlass:
-
macroporous glass 200 Å
- ELISA:
-
enzyme-linked immunosorbent assay
- HPLC:
-
high-performance liquid chromatography
- AIBN:
-
azodiisobutyronitrile
- BSA:
-
bovine serum albumin
- DMG:
-
3,6-di-O-methyl-d-glucose
- TLC:
-
thin-layer chromatography
- DMF:
-
dimethylformamide
- DMSO:
-
dimethylsulfoxide
- RI:
-
refractive index
- PBS:
-
phosphate buffered saline (0.14m NaCl, 0.01m sodium phosphate, pH 7.3)
References
Stowell CP, Lee YC (1980)Adv Carbohydr Chem Biochem 37:225–81.
Tang TW, Feizi T (1987)Carbohydr Res 161:133–43.
Chernyak AYa, Antonov KV, Kochetkov NK, Padyukov LN, Tsvetkova NV (1985)Carbohydr Res 141:199–212.
Kallin E, Lonn H, Norberg T, Elofsson M (1989)J Carbohydr Chem 8:597–611.
Bergelson LD, Dyatlovitskaya EV, Molotkovski YuG, Batrakov SG, Barsukov LI, Prokasova NV (1981) inPreparative Biochemistry of Lipids, p. 141. Moscow: Nauka.
Yurovskii VV, Bovin NV, Safonova NG, Vasilov RG, Khorlin AYa (1986)Soviet J Bio-org Chem 12:37–42.
Khorlin AYa, Bovin NV (1985)Soviet J Bio-org Chem 11:370–2.
Bovin NV, Ivanova IA, Khorlin AYa (1985)Soviet J Bio-org Chem 11:362–70.
Zemlyakov AE, Kakhayan ES, Chirva VYa (1989)Bioorg Khim 15:1527–32.
Byramova NE, Mochalova LV, Belyanchikov IM, Matrosovich MN, Bovin NV (1991)J Carbohydr Chem 10:691–700.
Korchagina EYu, Bovin NV (1992)Bioorg Khim 18:283–98.
Byramova NE, Bovin NV (1992)Proceedings of XVI International Carbohydrate Symposium, p. 209, France.
Horejsi V, Ticha M, Novotny J, Kocourek J (1980)Biochim Biophys Acta 623:439–48.
Stahl GL, Walter R, Smith CW (1978)J Org Chem 43:2285–6.
Bovin NV, Zemlyanukhina TV, Chagiashvili CN, Khorlin AYa (1988)Khim Prirodn Soed 6:777–85.
Ivanov AE, Kozlov LV, Shojbonov BB, Zubov VP, Antonov VK (1991)Biomed Chromatogr 5:90–3.
Hakomori S, Patterson CM, Nudelman E, Sekiguchi K (1983)J Biol Chem 258:11819–22.
Roy R, Tropper F (1988)Glycoconjugate J 5:203–6.
Roy R, Laferriere CA (1988)Carbohydr Res 177:C1-C4.
Galanina OE, Deryugina EI, Lapenkov MI, Nosyrev AE, Korchagina EYu, Zemlyanukhina TV, Bovin NV (1991)Bioorg Khim 17:343–52.
Bovin NV, Zurabyan SE, Khorlin AYa (1981)Proceedings of the XIIth Mendelev Congress on Comprehensive and Applied Chemistry (USSR), p. 120, Moscow: Nauka.
Matrosovich MN, Mochalova LV, Marinina VP, Byramova NE, Bovin NV (1990)FEBS Lett 272:209–12.
Tang PW, Gooi HC, Hardy M, Lee YC, Feizi T (1985)Biochem Biophys Res Commun 132:474–80.
Bankert RB, Mayers GL, Pressman D (1979)J Immunol 123:2466–74.
Parant M, Damias C, Audibert F, Parant F, Chedid L, Sache E, Lefrancier P, Choay J, Lederer E (1978)J Infect Dis 138:378–86.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bovin, N.V., Korchagina, E.Y., Zemlyanukhina, T.V. et al. Synthesis of polymeric neoglycoconjugates based onN-substituted polyacrylamides. Glycoconjugate J 10, 142–151 (1993). https://doi.org/10.1007/BF00737711
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00737711