Summary
-
1.
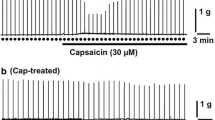
The effects of capsaicin applied to the exposed ventral surface of the medulla were studied on the mean arterial blood pressure, heart rate, respiration and sympathetic efferent nerve activity in chloralose-urethane-anaesthetized cats.
-
2.
The application of capsaicin produced a marked increase in the mean arterial blood pressure and sympathetic nerve activity, but not in the heart rate. The “intermediate area” proved to be the most sensitive to capsaicin. Pressor responses could be elicited repeatedly; tachyphylaxis was not noted provided a time interval 30 min elapsed between consecutive applications.
-
3.
Repeated applications of capsaicin at intervals of less than 30 min led to tachyphylaxis. However, pressor responses evoked by either topical application of glutamate or pentamethylene-tetrazole or bilateral carotid occlusion could invariably be demonstrated during this period of tachyphylaxis.
-
4.
Histological studies revealed the existence of a higherto unrecognized termination of capsaicin-sensitive nerve endings within the ventral medullary chemosensitive area of the cat.
-
5.
The results provide both functional and morphological evidence for the presence of a capsaicin-sensitive vasomotor mechanism in the ventral medullary chemosensitive area of the cat. It is suggested that the pressor effects of capsaicin applied to the ventral medullary chemosensitive area may be mediated by an activation of capsaicinsensitive primary sensory afferents terminating in this area. Accordingly, capsaicin-sensitive neuronal mechanisms located in the ventral medullary chemosensitive area may play an important role in the central nervous regulation of blood pressure.
Similar content being viewed by others
References
Amendt K, Czahurski J, Dembowsky K, Seller H (1978) Neurones within the “chemosensitive area” on the ventral surface of the brainstem which project of the intermediolateral column. Pflügers Arch 375:289–292
Ashton JH, Iwamoto GA, Longhurst JC, Mitchell JH (1982) Reflex cardiovascular depression induced by capsaicin injection into canine liver. Am J Physiol 242:H955-H960
Baraz LA, Khajutin VM, Molnár J (1965) Analysis of the stimulatory action of capsaicin on receptors and sensory fibres of the small intestine in the cat. Acta Physiol Hung 33:225–235
Berger AJ (1979) Distribution of carotid sinus nerve afferents to the solitary tract nuclei of the cat using transganglionic transport of horseradish peroxidase. Neurosci Lett 14:153–158
Bevan JA (1962) Action of lobeline and capsaicin on afferent endings in the pulmonary artery of the cat. Circulation Res 10:792–797
Bond SM, Cervero F, McQueen DS (1982) Influence of neonatally administered capsaicin on baroreceptor and chemoreceptor reflexes in the adult rat. Br J Pharmacol 77:517–524
Brender D, Webb Peploe MM (1969) Vascular responses to stimulation of pulmonary and carotid baroreceptors by capsaicin. Am J Physiol 217:1837–1845
Coleridge HM, Coleridge JCG, Kidd C (1964) Role of the pulmonary arterial baroreceptors in the effects produced by capsaicin in the dog. J Physiol 170:272–285
Coleridge HM, Coleridge JCG, Dangel A, Kidd C, Luck JC, Sleight P (1973) Impulses in slowly conducting vagal fibers from afferent endings in the veins, atria, and arteries of dogs and cats. Circ Res 33:87–97
Cottle MK (1964) Degeneration studies of primary afferents of IXth and Xth cranial nerves in the cat. J Comp Neurol 122:329–345
Crayton SC, Mitchell JH, Payne FC (1981) Reflex cardiovascular response during injection of capsaicin into skeletal muscle. Am J Physiol 240:H315-H319
Cuello AC, Gamse R, Holzer P, Lembeck F (1981) Substance P immunoreactive neurons following neonatal administration of capsaicin. Naunyn-Schmiedeberg's Arch Pharmacol 315:185–194
Daly M de B, Scott MJ (1958) The effects of stimulation of the carotid body chemoreceptors on heart rate in the dog. J Physiol (Lond) 144:148–166
Donnerer J, Lembeck F (1982) Analysis of the effects of intravenously injected capsaicin in the rat. Naunyn-Schmiedeberg's Arch Pharmacol 320:54–57
Eager RP (1970) Selective staining of degenerating axons in the central nervous system by a simplified silver method: spinal cord projections to external cuneate and inferior olivary nuclei in the cat. Brain Res 22:137–141
Errington ML, Dashwood MR (1979) Projections to the ventral surface of the cat brainstem demonstrated by horseradish peroxidase. Neurosci Lett 12:153–158
Farlow DM, Goodchild AK, Dampney RAL (1984) Evidence that vasomotor neurons in the rostral ventrolateral medulla project to the spinal sympathetic outflow via the dorsomedial pressor area. Brain Res 298:313–320
Feldberg W (1976) The ventral surface of the brain stem: a scarcely explored region of pharmacological sensitivity. Neuroscience 1:427–441
Feldberg W, Guertzenstein P (1984) Blood pressure effects of leptazol applied to the ventral surface of the brain stem of anaesthetized cats. J Physiol (Lond) 350:44P
Fitzgerald M (1983) Capsaicin and sensory neurons. A review. Pain 15:109–130
Fuxe K, Agnati LF, Rosell S, Harfstrand A, Folkers K, Lundberg JM, Andersson K, Hökfelt T (1982) Vasopressor effects of substance P and C-terminal sequences after intracisternal injection to α-cloralose anaesthetized rats; blockade by a substance P antagonist. Eur J Pharmacol 77:171–176
Gamse R, Molnár A, Lembeck F (1979) Substance P release from spinal cord slices by capsaicin. Life Sci 25:629–639
Gamse R, Holzer P, Lembeck F (1980) Decrease of substance P in primary afferent neurones and impairment of neurogenic plasma extravasation by capsaicin. Br J Pharmacol 68:207–213
Gamse R, Lackner D, Gamse G, Leeman SE (1981) Effect of capsaicin pretreatment on capsaicin evoked release of immunoreactive somatostatin and substance P from primary sensory neurons. Naunyn-Schmiedeberg's Arch Pharmacol 316:38–41
Gamse R, Petsche U, Lembeck F, Jancsó G (1982) Capsaicin applied to peripheral nerve inhibits axoplasmic transport of substance P and somatostatin. Brain Res 239:447–462
Göres E, Jung F (1959) Reizung von Gefäßreceptoren durch capsaicin. Acta Biol Med Germ 3:41–45
Guertzenstein PG (1973) Blood pressure effects obtained by drugs applied to the ventral surface of the brain stem. J Physiol (Lond) 229:395–408
Guertzenstein PG, Silver A (1974) Fall in blood pressure produced from discrete regions of the ventral surface to the medulla by glycine and lesions. J Physiol (Lond) 242:489–503
Haeusler G, Osterwalder R (1980) Evidence suggesting a transmitter or neuromodulatory role for substance P at the first synapase of the baroreceptor reflex. Naunyn-Schmiedeberg's Arch Pharmacol 314:111–121
Hajós M, Obál F, Jr, Jancsó G, Obál F (1983) The capsaicin sensitivity of the preoptic region is preserved in adult rats pretreated as neonates, but lost in rats pretreated as adults. Naunyn-Schmiedeberg's Arch Pharmacol 324:219–222
Helke CJ (1982) Neuroanatomical localisation of substance P: Implications for central cardiovascular control. Peptides 3:479–483
Helke CJ, Neil JJ, Massari VJ, Loewy AD (1982) Substance P neurons project from the ventral medulla to the intermediolateral cell column and ventral horn in the rat. Brain Res 243:147–152
Hökfelt T, Lundberg JM, Terenius L, Jancsó G, Kimmel J (1981) Avian pancreatic polypeptide (AAP) Immunoreactive neurons in the spinal cord and spinal trigeminal nucleus. Peptides 2: 81–87
Jancsó G (1981) Chemosensitive primary sensory neurons: some morphological and functional characteristics. In: Brown AG, Réthelyi M (eds) Spinal cord sensations. Scottish Academic Press, Edinburgh, pp 55–56
Jancsó G, Such G (1983) Effects of capsaicin applied perineurally to the vagus nerve on cardiovascular and respiratory functions in the cat. J Physiol 341:359–370
Jancsó G, Király E, Jancsó-Gábor A (1977) Pharmacologically induced selective degeneration of chemosensitive primary sensory neurones. Nature (Lond) 270:741–743
Jancsó G, Király E, Jancsó-Gábor A (1980a) Chemosensitive pain fibres and inflammation. Int J Tiss Reac 2:57–66
Jancsó G, Király E, Jancsó-Gábor A (1980a) Direct evidence for an axonal site of action of capsaicin. Naunyn-Schmiedeberg's Arch Pharmacol 313:91–94
Jancsó G, Hökfelt T, Lundberg JM, Király E, Halász N, Nilsson G, Terenius L, Rehfeld J, Steinbusch H, Verhofstad A, Elde R, Said S, Brown M (1981) Immunohistochemical studies on the effect of capsaicin on spinal and medullary peptide and monoamine neurons using antisera to substance P, gastrin/CCK, somatostatin, VIP, enkephalin, neurotensin and 5-hydroxytryptamine. J Neurocytol 10:963–980
Jessel TM, Iversen LL, Cuello AC (1978) Capsaicin-induced depletion of substance P from primary sensory neurones. Brain Res 152:183–188
Kalia M, Mesulam MM (1980) Brain stem projections of sensory and motor components of the vagus complex in the cat: 1. The cervical vagus and nodose ganglion. J Comp Neurol 193:435–465
Keeler JR, Shults CW, Chase TN, Helke CJ (1984) The ventral surface of the medulla in the rat: Pharmacologic and autoradiographic localization of GABA-induced cardiovascular effects. Brain Res 297:217–224
Kille JF, Schlaefke ME (1978) Histological studies on the medullary chemosensitive areas after chronic denervation of a carotid body. Neurosci Lett Suppl 1:S16
Kollai M, Koizumi K, Brooks C McC (1978) Nature differential sympathetic discharges in chemoreceptor reflexes. Proc Natl Acad Sci USA 75:5239–5243
Ljungdahl A, Hökfelt T, Nilsson G (1978) Distribution of substance P-like immunoreactivity in the central nervous system of the rat. I. Cell bodies and nerve terminals. Neuroscience 3:861–943
Loeschcke HH (1982) Central chemosensitivity and the reaction theory. J Physiol (Lond) 332:1–24
Loeschcke HH, Delattre J, Schläfke M, Trough CO (1970) Effects on respiration and circulation of electrically stimulating the ventral surface of the medulla oblongata. Respirat Physiol 10:184–197
Longhurst JC, Ashton JA, Iwamoto GA (1980a) Cardiovascular reflexes resulting from capsaicin-stimulated gastric receptors in anesthetized dogs. Circ Res 46:780–788
Longhurst JC, Mitchell JH, Moore MB (1980b) The spinal cord ventral root: an afferent pathway of the hind-limb pressor reflex in cats. J Physiol (Lond) 301:467–476
Mantyh PW, Hunt SP (1984) Evidence for cholecystokinin-like immunoreactive neurons in the rat medulla oblongata which project to the spinal cord. Brain Res 291:49–54
McAllen RM, Neil JJ, Loewy AD (1982) Effects of kainic acid applied to the ventral surface of the medulla oblongata on vasomotor tone, the baroreceptor reflex and hypothalamic autonomic responses. Brain Res 238:65–76
Mitchell RA, Loeschcke HH, Severinghaus JW, Richardson BW, Massion WH (1963) Regions of respiratory chemosensitivity on the surface of the medulla. Ann NY Acad Sci 109:661–681
Nagy JI, Vincent SR, Staines WA, Fibiger HC, Reisine TD, Yamamura HI (1980) Neurotoxic action of capsaicin on spinal substance P neurones. Brain Res 186:435–444
Nagy JI, Hunt SP, Iversen LL, Emson PC (1981) Biochemical and anatomical observations on the degeneration of peptidecontaining primary afferent neurons after neonatal capsaicin. Neuroscience 6:1923–1934
Neil E, Redwood, CRM, Schweitzer A (1949) Pressor responses to electrical stimulation of the carotid sinus nerve in cats. J Physiol (Lond) 109:259–271
Palermo NN, Brown HK, Smith DL (1981) Selective neurotoxic action of capsaicin on glomerular C-type terminals in rat substantia gelatinosa. Brain Res 208:506–510
Petty M, Reid J (1982) The cardiovascular effects of centrally administered substance P in the anesthetized rabbit. Eur J Pharmacol 82:9–14
Pórszász J, György L, Pórszász-Gibiszer K (1955) Cardiovascular and respiratory effects of capsaicin. Acta Physiol Hung 8:61–76
Pórszász J, Such G, Pórszász-Gibiszer K (1957) Circulatory and respiratory chemoreflexes. I. Analysis of the site of action and receptor types of capsaicin. Acta Physiol Hung 12:189–205
Quest JA, Dias-Soza J, Norman WP, Holtman JR, Jr, Gillis RA (1984) Central nervous system site of action of capsaicin-induced cardiovascular changes in the cat. J Pharmacol Exp Ther 228:719–724
Ribeiro-da-Silva A, Coimbra A (1984) Capsaicin causes selective damage to type I synaptic glomeruli in rat substantia gelationsa. Brain Res 290:380–383
Rózsa Zs, Jancsó G, Varró V (1984) Possible involvement of capsaicin-sensitive sensory nerves in the regulation of intestinal blood flow in the dog. Naunyn-Schmiedeberg's Arch Pharmacol 326:352–356
Schläfke M, Loeschcke HH (1967) Lokalisation eines an der Regulation von Atmung und Kreislauf beteiligten Gebietes an der ventralen Oberfläche der Medulla oblongata durch Kälteblockade. Pflügers Arch 297:201–220
Such G, Jancsó G (1984) Axonal effects of capsaicin: an electrophysiological study. Acta Physiol Hung (in press)
Szolcsányi J (1982) Capsaicin type pungent agents producing pyrexia. In: Milton AS (ed) Handbook of experimental pharmacology, vol. 60. Springer, Berlin Heidelberg New York, pp 437–478
Szolcsányi J, Joó F, Jancsó-Gábor A (1971) Mitochondrial changes in preoptic neurones after capsaicin desensitization of the hypothalamic thermoreceptors in rats. Nature 229:116–117
Takano Y, Martin JE, Leeman SE, Loewy AD (1984) Substance P immunoreactivity released from rat spinal cord after kainic acid excitation of the ventral medulla oblongata: a correlation with increases in blood pressure. Brain Res 291:168–172
Theriault E, Otsuka M, Jessel T (1979) Capsaicin-evoked release of substance P from primary sensory neurons. Brain Res 170:209–213
Toda N, Usui H, Nishino N, Fujiwara M (1972) Cardiovascular effects of capsaicin in dogs and rabbits. J Pharmacol Exp Ther 181:512–521
Toh CC, Lee TS, Kiang AC (1955) The pharmacological action capsaicin and analogs. Br J Pharmacol 10:175–182
Trouth CO, Loeschcke HH, Berndt J (1973a) A superficial substrate on the ventral surface of the medulla oblongata influencing respiration. Pflügers Arch 339:135–152
Trouth CO, Loeschcke HH, Berndt J (1973b) Topography of the circulatory responses to electrical stimulation in the medulla oblongata. Relationship to respiratory responses. Pflügers Arch 339:185–201
Willette RN, Barcas PP, Krieger AJ, Sapru HN (1983) Vasopressor and depressor areas in the rat medulla. Identification by microinjection ofl-glutamate. Neuropharmacology 22:1071–1079
Williford DJ, Hamilton BL, Dias Souza J, Williams TP, Dimicco Ja, Gillis RA (1980) GABAergic mechanisms influencing arterial pressure and heart rate in the cat. Circ Res 47:80–88
Yaksh TL, Jessel TM, Gamse R, Mudge AW, Leeman SE (1980) Intrathecal morphine inhibits substance P release from mammalian spinal cord, in vivo. Nature (Lond) 286:155–157
Yamada KA, McAllen RM, Loewy AD (1984) GABA antagonists applied to the ventral surface of the medulla oblongata block the baroreceptor reflex. Brain Res 297:175–180
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Jancsó, G., Such, G. Evidence for a capsaicin-sensitive vasomotor mechanism in the ventral medullary chemosensitive area of the cat. Naunyn-Schmiedeberg's Arch. Pharmacol. 329, 56–62 (1985). https://doi.org/10.1007/BF00695193
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00695193