Abstract
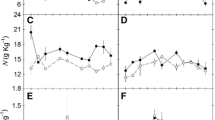
Leaf tolerance to high temperatures, as determined by electrolyte leakage and chlorophyll a fluorescence, was compared for Artemisia tridentata (Asteraceae), a widespread shrub of the Great Basin, Colorado Plateau, and western slope of the Rocky Mountains, and Potentilla gracilis (Rosaceae), a herbaceous forb common to high-elevation meadows of the western United States. Species-specific and treatment-specific differences in leaf temperature, high-temperature tolerance and chlorophyll a fluorescence from photosystem II were compared, to test the hypothesis that plants at ecosystem borders will exhibit species-specific responses to climate change. Measurements were made for plants exposed to a climate change warming manipulation on a major ecosystem border at the Rocky Mountain Biological Laboratory, Colorado, United States, in July and August 1995. In July, daily maximal leaf temperatures were significantly higher for P. gracilis than for A. tridentata. Leaf temperatures were slightly lower in August than July for leaves of both species, on control and heated plots, despite the fact that daily maximum air temperatures were not significantly different for the two months. High-temperature tolerance was determined for leaves treated for 1 h at temperatures ranging from 15°C to 65°C. LT50 was approximately 46°C for both species on control plots, but was 43°C for leaves of both species from heated plots, contrary to the predictions of the hypothesis. No shift in LT50 (acclimation) was apparent between July and August. Changes in chlorophyll a fluorescence from photosystem II (F V /F M ) were used to characterize the photosynthetic response to high temperatures. For both A. tridentata and P. gracilis in July, F V /F M was about 0.7, but decreased for temperatures above 40°C. The results suggest that plant responses to global warming at ecosystem borders may be influenced by factors other than leaf-level physiological tolerance to elevated temperatures.
Similar content being viewed by others
References
Adams WW III, Demmig-Adams B (1994) Carotenoid composition and down regulation of photosystem II in three conifer species during the winter. Physiol Plant 92:451–458
Allen-Diaz B (1991) Water table and plant species relationships in Sierra Nevada meadows (California, USA). Am Midl Nat 126:30–43
Berry JA, Björkman O (1980) Photosynthetic response and adaptation to temperature in higher plants. Annu Rev Plant Physiol 31:491–543
Björkman O, Badger MR, Armond PA (1980) Response and adaptation of photosynthesis to high temperatures. In: Turner NC, Kramer PJ (eds) Adaptation of plants to water and high temperature stress. Wiley, New York, pp 233–249
Caldwell MM (1985) Cold desert. In: Chabot B, Mooney H (eds.) Physiological ecology of North American plant communities. Chapman and Hall, London, pp 198–212
Didden-Zopfy B, Nobel PS (1982) High temperature tolerance and heat acclimation of Opuntia bigelovii. Oecologia 52:176–180
Ehleringer JR (1980) Leaf morphology and reflectance in relation to water and temperature stress. In: Turner NC, Kramer PJ (eds) Adaptation of plants to water and high temperature stress. Wiley, New York, pp 295–308
Evans RD, Black RA, Link SO (1990) Rehydration-induced changes in pressure-volume relationships of Artemisia tridentata Nutt. ssp. tridentata. Plant Cell Environ 13:455–461
Galen C, Stanton M (1993) Short-term responses of alpine buttercups to experimental manipulations of growing season length. Ecology 74:1052–1058
Galen C, Stanton M (1995) Responses of snowbed plant species to changes in growing-season length. Ecology 79:1546–1557
Gamon JA, Pearcy RW (1989) Leaf movement, stress avoidance and photosynthesis in Vitis californica. Oecologia 79:475–481
Gamon JA, Pearcy RW (1990) Photoinhibition in Vitis californica: interactive effects of sunlight, temperature and water status. Plant Cell Environ 13:267–275
Gates DM (1993) Climate change and its biological consequences. Sinauer, Sunderland
Grace J, Norton DA (1990) Climate and growth of Pinus sylvestris at its upper altitudinal limit in Scotland: evidence from tree growth-rings. J Ecol 78:601–610
Harte J, Shaw MR (1995) Shifting dominance within a montane vegetation community: results of a climate-warming experiment. Science 267:876–880
Harte J, Torn MS, Chang F, Feifarek B, Kinzig A, Shaw R, Shen K (1995) Global warming and soil microclimate: results from a meadow-warming experiment. Ecol Appl 5:132–150
Havaux M (1992) Stress tolerance of photosystem II in vivo. Antagonistic effects of water, heat, and photoinhibition stresses. Plant Physiol 100:424–432
Hoffman AA, Blows MW (1994) Species borders: ecological and evolutionary perspectives. Trends Ecol Evol 9:223–227
Houghton JT, Bolin B (1992) Intergovernmental panel on climate change. United Nations Environment Programme/World Meteorological Organization, Geneva
Jacubos B, Romme WH (1993) Invasion of subalpine meadows by lodgepole pine in Yellowstone National Park, Wyoming, U.S.A. Arct Alp Res 4:382–390
Larcher W (1995) Physiological plant ecology, 3rd edn. Springer, Berlin Heidelberg New York
Leishman MR, Hughes L, French K, Armstrong D, Westoby M (1992) Seed and seedling biology in relation to modelling vegetation dynamics under global climate change. Aust J Bot 40:599–613
Levitt J (1980) Responses of plants to environmental stresses, vol 1. Chilling, freezing, and high temperature stresses, 2nd edn. Academic Press, New York
Loik ME, Nobel PS (1993) Freezing tolerance and water relations of Opuntia fragilis from Canada and the United States. Ecology 74:1722–1732
Manabe S, Wetherald RT (1986) Reduction in summer soil wetness induced by an increase in atmospheric carbon dioxide. Science 232:626–628
Melcher PJ, Goldstein G, Meinzer FC, Minyard B, Giambelluca TW, Lopeer LL (1994) Determinants of thermal balance in the Hawaiian giant rosette plant, Agyroxiphium sandwicense. Oecologia 98:412–418
Mooney HA, Ehleringer J, Björkman O (1977) The energy balance of leaves of the evergreen desert shrub Atriplex hymenelytra. Oecologia 29:301–310
Nobel PS (1984) Extreme temperatures and the thermal tolerances for seedlings of desert succulents. Oecologia 62:310–317
Nobel PS, Geller GN, Kee SC, Zimmerman AD (1986) Temperatures and thermal tolerances for cacti exposed to high temperatures near the soil surface. Plant Cell Environ 9:279–287
Peters RL, Lovejoy TE (1992) Global warming and biological diversity. Yale University Press, New Haven
Richardson DM, Bond WJ (1991) Determinants of plant distribution: evidence from pine invasions. Am Nat 137:639–668
Schneider SH (1989) The greenhouse effect: science and policy. Science 243:771–781
Smith SD, Nowak RS (1990) Ecophysiology of plants in the Intermountain lowlands. In: Osmond CB, Pitelka LF, Hidy GF (eds) Plant biology of the Basin and Range. Springer, Berlin Heidelberg New York
Smith SD, Didden-Zopfy B, Nobel PS (1984) High-temperature responses of North American cacti. Ecology 65:643–651
Smith WK, Nobel PS (1977) Temperature and water relations for sun and shade leaves of a desert broadleaf, Hyptis emoryi. J Exp Bot 28:169–183
Weber WA (1976) Rocky Mountain flora: western slope. Colorado Associated University Press, Boulder
Woodward FI, Williams BG (1987) Climate and plant distribution at global and local scales. Vegetatio 69:189–197
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Loik, M.E., Harte, J. High-temperature tolerance of Artemisia tridentata and Potentilla gracilis under a climate change manipulation. Oecologia 108, 224–231 (1996). https://doi.org/10.1007/BF00334645
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00334645