Summary
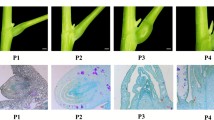
We isolated a flower-specific cDNA, FST (flower-specific thionin), which encodes a novel thionin from tobacco. Thionins are basic and cysteine (Cys)-rich, low molecular weight proteins found in many plants. They are believed to play a role in plant defense against pathogens. The central domain of the FST protein shares homology with three γ-thionins. Like other thionin precursors, the FST protein has an N-terminal domain characteristic of a signal peptide and an acidic C-terminal domain. FST mRNA accumulates specifically in developing flowers and its level drops as flowers mature. Transcripts are present in petals, stamens and pistil but are not detectable in sepals. In situ hybridization revealed that FST mRNA is most abundant in the epidermal cells along the adaxial surface of petals, and in the surface cell layers of the carpel and anther walls. If the FST protein indeed has a protective role in flowers, this pattern of spatial distribution of FST mRNA would appear to maximize this effect on the two internal reproductive whorls. A possible biological role for FST is discussed.
Similar content being viewed by others
References
Apel K, Bohlmann H, Reimann-Philipp U (1990) Leaf thionins, a novel class of putative defense factors. Physiol Plantarum 80:315–321
Bernier G (1988) The control of floral evocation and morphogenesis. Annu Rev Plant Physiol Mol Biol 39:175–219
Bohlmann H, Apel K (1987) Isolation and characterization of cDNAs coding for leaf-specific thionins closely related to the endosperm-specific hordothionin of barley (Hordeum vulgare L.) Mol Gen Genet 207:446–454
Bohlmann H, Apel K (1991) Thionins. Annu Rev Plant Physiol 42:227–240
Bohlmann H, Clausen S, Behnke S, Giese H, Hiller C, Reimann-Philipp U, Schrader G, Barkholt V, Apel K (1988) Leaf-specific thionins of barley — a novel class of cell wall proteins toxic to plant-pathogenic fungi and possibly involved in the defence mechanism of plants. EMBO J 7:1559–1565
Carrasco L, Vazquez D, Hernandez-Lucas C, Carbonero P, Garcia-Olmedo F (1981) Thionins: Plant peptides that modify membrane permeability in cultured mammalian cells. Eur J Biochem 116:185–189
Church G, Gilbert W (1984) Genomic sequencing. Proc Natl Acad Sci 81:1991–1995
Clore GM, Nilges M, Sukumaran DK, Brunger AT, Karplus M, Gronenborn AM (1986) The three-dimensional structure of α1-purothionin in solution: combined use of nuclear magnetic resonance, distance geometry and restrained molecular dynamics. EMBO J 5:2729–2735
Coen E (1991) The role of homeotic genes in flower development and evolution. Annu Rev Plant Physiol Mol Biol 42:241–279
Colilla FJ, Rocher A, Mendez E (1990) γ-Purothionins: amino acid sequence of two polypeptides of a new family of thionins from wheat endosperm. FEBS Lett 270:191–194
Ebraim-Nesbat F, Behnke S, Kleinhofs A, Apel K (1989) Cultivar-related differences in the distribution of cell-wall-bound thionins in compatible and incompatible interactions between barley and powdery mildew. Planta 179:203–210
Fraser RSS (1981) Evidence for the occurrence of the “pathogenesis-related” proteins in leaves of healthy tobacco plants during flowering. Physiol Plant Path 19:69–76
Garcia-Olmedo F, Rodriguez-Pelenzuela P, Hernandez-Lucas C, Ponz F, Marana C, Carmona MJ, Lopez-Fando J, Fernandez JA, Carbonero P (1989) The thionins: A protein family that includes purothionins, viscotoxins and crambins. Oxford Surv Plant Mol Cell Biol 6:31–60
Gasser CS (1991) Molecular studies on the differentiation of floral organs. Annu Rev Plant Physiol Mol Biol 42: 621–649
Gausing K (1987) Thionin genes specifically expressed in barley leaves. Planta 171:241–246
Goldberg RB (1988) Plants: Novel developmental processes. Science 240:1460–1467
von Heijne G (1985) Signal sequences: The limits of variation. J Mol Biol 184:99–105
von Heijne G (1986) A new method for predicting signal sequence cleavage sites. Nucleic Acids Res 14:4683–4690
Hendrickson WA, Teeter MM (1981) Structure of the hydrophobic protein crambin determined directly from the anomalous scattering of sulphur. Nature 290:107–113
Huynh VT, Young RA, Davis RW (1985) Constructing and screening cDNA libraries in gt10 and gt11. In: DNA cloning. A practical approach, vol I. IRL Press, Oxford, Washington DC, pp 49–78
Ishibashi N, Yamauchi D, Minamikawa T (1990) Stored mRNA in cotyledons of Vigna unguiculata seeds: nucleotide sequence of cloned cDNA for a stored mRNA and induction of its synthesis by precocious germination. Plant Mol Biol 15:59–64
Joshi CP (1983) Putative polyadenylation signals in nuclear genes of higher plants: a compilation and analysis. Nucleic Acids Res 15:9627–9640
Kelly AJ, Zagotta MT, White RA, Chang C, Meeks-Wagner DR (1990) Identification of genes expressed in the tobacco shoot apex during the floral transition. Plant Cell 2:963–972
Koltunow AM, Truetlner J, Cox KH, Wallroth M, Goldberg RB (1990) Different temporal and spatial gene expression patterns occur during anther development. Plant Cell 2:1201–1224
Langdale JA, Rothermel B, Nelson T (1988) Cellular pattern of photosynthetic gene expression in developing maize leaves. Genes Dev 2:106–115
Lotan T, Ori N, Fluhr R (1989) Pathogenesis-related proteins are developmentally regulated in tobacco flowers. Plant Cell 1: 881–887
Maniatis T, Fritsch EF, Sambrook J (1982) Molecular cloning: A laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York
McHale NA, Kawata EE, Cheung AY (1990) Plastid disruption in a thiamine-requiring mutant of Nicotiana sylvestris blocks accumulation of specific nuclear and plastid mRNAs. Mol Gen Genet 221:203–209
Melzer S, Majewski DM, Apel K (1990) Early changes in gene expression during the transition from vegetative to generative growth in the long-day plant Sinapis alba. Plant Cell 2:953–961
Mendez E, Moreno A, Colilla F, Pelaez F, Limas GG, Mendez R, Soriano F, Salinas M, de Haro C (1990) Primary structure and inhibition of protein synthesis in eukaryotic cell-free system of a novel thionin, y-hordothionin, from barley endosperm. Eur J Biochem 194:533–539
Neale AD, Wahleithner JA, Lund M, Bonnett HT, Kelly A, MeeksWagner DR, Peacock WJ, Dennis ES (1990) Chitinase, β-1, 3-glucanase, osmotin, and extensin are expressed in tobacco explants during flower formation. Plant Cell 2:673–684
Ponz F, Paz-Ares J, Hernandez-Lucas C, Garcia-Olmedo F, Carbonero P (1986) Cloning and nucleotide sequence of a cDNA encoding the precursor of the barley toxin α-hordothionin. Eur J Biochem 156:131–135
Rigden J, Coutts R (1988) Pathogenesis-related proteins in plants. Trends Genet 4:87–89
Stiekema WJ, Heidekamp F, Dirkse WG, van Beckum J, de Haan P, ten Bosch C, Louwerse JD (1988) Molecular cloning and analysis of four potato tuber mRNAs. Plant Mol Biol 11:255–269
Steinmuller K, Apel K (1986) A simple and efficient procedure for isolating plant chromatin which is suitable for studies of DNAse I-sensitive domains and hypersensitive sites. Plant Mol Biol 7:87–94
Author information
Authors and Affiliations
Additional information
Communicated by J. Schell
Rights and permissions
About this article
Cite this article
Gu, Q., Kawata, E.E., Morse, M.J. et al. A flower-specific cDNA encoding a novel thionin in tobacco. Molec. Gen. Genet. 234, 89–96 (1992). https://doi.org/10.1007/BF00272349
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00272349