Summary
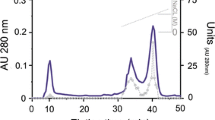
The digestive glands of many marine molluscs are rich sources of arylsufatase enzymes which may function in the catabolism of sulfated polysaccharides in the diets of herbivorous species. Arylsulfatases, partially purified from the hepatopancreas of the red abalone, Haliotis rufescens, were investigated with respect to heterogeneity, catalytic requirements, and timing of induction during development. Four hepatopancreatic enzymes were purified from adult animals using a combination of hydrophobic interaction and anion-exchange chromatography. Zymograms of the four partially-purified enzymes produced by electrophoresis under nondenaturing conditions revealed a fifth, relatively more basic isozyme. All four partially-purified enzymes appear to be monomeric, with molecular weights of approximately 43 000 Da each, as measured by gel filtration. The affinities for p-nitrocatechol sulfate, pH optima, and strengths of inhibition by anions displayed by these enzymes are similar to the values reported for other molluscan arylsulfatases. Three of the four enzymes have K m values between 0.8 and 2.0 mM for p-nitrocatechol sulfate; the remaining enzyme (A2) has a K m of 6.7 mM. All four enzymes have pH and temperature optima of 5.5 and 45°C, respectively. Three of the four enzymes have-t1/2(50°C) values of 3.5 min; the enzyme A4 has a t1/2 has a t1/2(50°C) of 8.5 min. A monoclonal antibody directed against form A1b does not cross react with any of the other hepatopancreatic arylsulfatases when assayed by Western blot, confirming the structural heterogeneity of the adult enzymes.
Total arylsulfatase activity increases in a biphasic manner during early abalone development, with the first increase occurring early in larval maturation. The secoad phase of enzyme expression is dependent upon the induction of settlement and metamorphosis of the competent veliger larvae, strongly suggesting that the expression of arylsulfatase synthesis (and the maturation of the digestive gland, the hepatopancreas) is controlled by genetic events which occur as a result of metamorphosis. Competent veliger larvae express only two arylsulfatase forms, which share many physicochemical and kinetic characteristics with the adult hepatopancreatic enzymes. However, neither of the larval arylsulfatases is recognized by the monoclonal antibody to form A1b from adult hepatopancreas. Endogenous enzyme inhibitor levels in larvae remain constant throughout the period of arylsulfatase induction, and therefore do not contribute to the control of arylsulfatase activity levels during development.
These results are the first documentation of the developmental induction of a specific protein(s) in abalone as a result of metamorphosis. The significance of the timing of arylsulfatase expression is discussed in relation to potential physiological substrates and the dietary switching which occurs at metamorphosis. Possible genetic events which are consistent with the observed patterns of expression of these enzymes also are considered.
Similar content being viewed by others

Abbreviations
- BSA :
-
bovine serum albumin
- C :
-
centigrade
- Da :
-
daltons
- DEAE :
-
diethylaminoethyl
- ELISA :
-
enzyme-linked immunosorbent assay
- FPLC :
-
fast protein and polynucleotide liquid chromatography
- GABA :
-
γ-aminobutyric acid
- HAT:
-
hypoxanthine, aminopterin, thymidine
- Hepes:
-
N-(2-Hydroxythyl)piperazine-N'-(2-ethanesulfonic acid)
- HPLC :
-
high pressure liquid chromatography
- KBS :
-
Kantor's balanced salt solution
- K m :
-
Michaelis constant
- PBS :
-
phosphate buffered saline
- R m :
-
relative mobility
- RPMI :
-
Roswell Park Memorial Institute
- SDS :
-
sodium dodecyl sulfate
- T :
-
time
- TBS :
-
TRIS buffered saline
- V max :
-
maximal velocity
References
Agogbua S, Anosike E, Ugochukwu E (1978) Partial purification and some properties of arylsulfatases from the gut of the giant African snail, Achatina achatina. Comp Biochem Physiol 59B:169–173
Akasaka K, Terayama H (1983) A proteoglycan fraction isolated from the EDTA extract of sea urchin (Hemicentrotus pulcherrimus) gastrulae stimulates reaggregation of dissolved embryonic cells. Exp Cell Res 150:226–233
Banna H (1980) Histochemical studies of some enzymes in the tissues of the schistosome vector snail Balinus truncatus (Aoduin) with special reference to the effects of a molluscicide. I. Hydrolases. Histochem J 12:145–152
Bolognani L, Masserini M, Bodini PA, Bolognani-Fantin AM, Ottaviani E (1981) Lipid composition in ganglia of Mollusca. J Neurochem 36:821–825
Bradford M (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Campbell AM (1984) Monoclonal antibody technology. Elsevier, Amsterdam Cariolou MA, Morse DE (1988) Purification and characterization of calcium-binding conchiolin shell peptides from the mollusc, Haliotis rufescens, as a function of development. J Comp Physiol B 157:717–730
Christomanou H, Sandoff K (1977) A sensitive fluorescence assay for the simulatneous and separate determination of arylsulfatases A and B. Clin Chim Acta 79:527–529
Collier JR (1966) The transcription of genetic information in the spiralian embryo. Curr Top Dev Biol 1:39–59
Collier JR (1983) The biochemistry of molluscan development. In: Verdonk NH, van den Biggelaar JAM, Tompa AS (eds) The Mollusca, vol. 3. Academic Press, New York
Corner E (1960) Steroid sulphatase, arylsulphatase and β-glucuronidase in marine invertebrates. J Mar Biol Assoc UK 39:51–61
Davidson EH (1986) IV. Differential gene function in the embryo. Gene activity in early development. Academic Press, New York
Dizik M, Elliott RW (1977) A gene apparently determining the extent of sialyzation of lysosomal α mannosidase in mouse liver. Biochem Genet 15:31–46
Dodgson KS (1959) Studies on sulphatases. Enzymologia 20:301–306
Dodgson KS, Powell GM (1959a) Studies on sulphatases. XXVI. Arylsulphatase activity in the digestive juice and digestive gland of Helix pomatia. Biochem 73:666–671
Dodgson KS, Powell GM (1959b) Studies on sulphatases XXVII. The partial purification and properties of the arylsulphatase of the digestive gland of Helix pomatia. Biochemistry 73:672–679
Dodgson KS, Spencer B (1952) Purification of the arylsulphatase of Patella vulgata. Proc Biochem Soc.
Dodgson KS, Lewis J, Spencer B (1953) Studies on sulphatases. III. The arylsulphatase and β-glucuronidase of marine molluscs. Biochem J 55:253–259
Dodgson KS, White G, Fitzgerald J (1982a) In: Sulfatases of microbial origin, vol 1. CRC Press, Boca Raton, Florida
Dodgson KS, White G, Fitzgerald J (1982b) In: Sulfatases of microbial origin vol 2. CRC Press, Boca Raton, Florida
Drewa G, Dabrowska T, Zukowska-Arendarczyk M, Zbytniewski Z, Pautsch F (1983) The influence of anionic detergent on the arylsulphatase activity in the shirmp Crangon crangon during the molting cycle. Pol Arch Hydrobiol 30:57–63
Engvall E, Perlman P (1971) Enzyme-linked immunosorbant assay (ELISA). Quantitative assay of immunoglobulin G. Immunochemistry 8:871–874
Farooqui A (1976) Effect of neuraminidase on the multiple forms of arylsulphatase B. Biochemie 58:759–761
Farooqui A, Roy AB (1976) The sulphase of ox liver. XX. The preparation of sulphatases B1α and B1β. Biochim Biophys Acta 452:431–439
Fedecka-Bruner B, Anderson M, Epel D (1971) Control of enzyme synthesis in early sea urchin development: arylsulfatase activity in normal and hybrid embryos. Dev Biol 25:655–671
Fretter V (1969) Aspects of metamorphosis in prosobranch molluscs. Proc Malacol Soc London 38:375–386
Glombitza K, Stoffelen H (1972) Antibiotics from alga. VI. 2,3-Dibromo-4,5-dihydroxybenzyl alcohol-4,5-bis (hydrogen sulfate) dipotassium salt from Rhodomelaceae. Planta Med 22:391–395
Harada T, Murooka Y (1980) Participation of tyramine oxidase in multiple control of bacterial arylsulfatase synthesis. Mem Inst Sci Ind Res (Osaka Univ) 37:45–67
Hatanaka H, Ogawa Y, Egami F (1974) O-Ascorbic acid sulfate esters with special reference to enzymatic hydrolysis. J Biochem 75:861–866
Hatanaka H, Ogawa Y, Egami F (1975) Copurification of O-ascorbate-2-sulfate sulfohydrolase and arylsulfatase activities from the liver of a marine gastropod, Charonia lampas. J Biochem 77:353–359
Hatanaka H, Ogawa Y, Egami F (1976a) Two glycosulfatases from the liver of a marine gastropod, Charonia lampas. J Biochem 79:27–34
Hatanaka H, Ogawa Y, Egami F (1976b) Arylsulphatase and glycosulphatase of Charonia lampas: substrate specificity towards sugar sulphate derivatives. Biochem J 159:445–448
Hatanaka H, Egami Ishizuka I, Nagai Y (1976) Sulphogalactolipid sulphohydrolase activity of arylsulphatase purified from a marine gastropod, Charonia lampas. Biochim Biophys Acta 438:176–185
Hoogenrad N, Helman T, Hoogenrad J (1983) The effect of pre-injection of mice with Pristane on ascites tumor formation and monoclonal antibody production. J Immunol Methods 61:317–328
Hoshi M, Moriya T (1980) Arylsulfatase of sea urchin sperm. 2. Arylsulfatase as a lysin of sea urchins. Dev Biol 74:343–350
Iga DI (1982) The study of arylsulphatasic activity in the mussel Mytilas galloprovinciallis. Rev Roum Biochim 19:215–219
Karn R, Rosenblum R, Ward J, Merritt A, Shulkin J (1974) Immunological relationships and post-translational modifications of human salivary amylase (amy1) and pancreatic amylase (amy2) isoezymes. Biochem Genet 12:485–499
Kohler G, Milstein C (1975) Continuous culture of fused cells secreting antibody of predefined specificity. Nature 256:495–497
Laemmli U (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Leon Y, Bulbrook R, Corner E (1960) Steroid sulphatase, arylsulphatase and β-glucuronidase in the Mollusca. Biochem J 75:612–647
Lineweaver H, Burk D (1934) The determination of enzyme dissociation constants. J Am Chem Soc 56:658–668
Littlefield JW (1964) Selection of hybrids from matings of fibroblasts in vitro and their presumed recombinants. Science 145:709–710
McCandles E, Craigie J (1979) Sulfated polysaccharides in red and brown algae. Ann Rev Plant Physiol 30:41–53
Merdsoy B, Farley J (1973) Phasic activity in the digestive gland cells of the marine prosobranch gastropod, Littorina littorea (L.). Proc Malacol Soc Lond 40:473–482
Moore M, Halton D (1977) The cytochemical localization of lysosomal hydrolases in the digestive cells of Littorinids and changes induced by larval trematode infection. Z Parasitenkd 53:115–122
Moore M, Pipe R, Farrar S (1982) Lysosomal and microsomal responses to environmental factors in Littorina littorea from Sullom Voe. Mar Poll Bull 13:340–345
Moriya T, Hoshi M (1980) Chracterization and partial purification of arylsulfatase from the seminal plasma of the sea urchin, Strongylocentrotus intermedius. Arch Biochem Biophys 201:216–223
Morrill JB, Norris E, Smith SD (1976) Electro-and immunoelectrophoretic patterns of egg albumen of the pond snail Limnaea stagnalis. Am Zool 16:547–562
Morse ANC, Morse DE (1984) Recruitment and metamorphosis of Haliotis larvae induced by molecules uniquely available at the surfaces of crustose red algae. J Exp Mar Biol Ecol 75:191–215
Morse DE, Duncan H, Hooker N, Morse A (1977) Hydrogen peroxide induces spawning in mollusks, with activation of prostaglandin endoperoxide synthetase. Science 196:298–300
Morse DE, Duncan H, Hooker N, Morse A (1978) An inexpensive chemical method for the control and synchronous induction of spawning and reproduction in molluscan species important as protein-rich food resources. In: Proceedings of the U.N. symposium, CICAR-II, Caracas, 1976; FAO Fish Rep 200:291–300
Morse DE, Hooker N, Duncan H, Jensen L (1979) γ-Aminobutyric acid, a neurotransmitter, induces planktonic abalone larvae to settle and begin metamorphosis. Science 204:407–410
Morse DE, Tegner M, Duncan H, Hooker N, Trevalyan G, Cameron A (1980) Induction of settling and metamorphosis of planktonic molluscan (Haliotis) larvae. III: Signaling by metabolites of intact algae is dependent on contact. In: Muller-Schwartz D, Silverstein RM (eds) Chemical signaling in vertebrate and aquatic animals. Plenum Press, New York, pp 67–86
Moss D (1982) Origins and structures of multiple forms of enzymes. In: Isoenzymes, Chapman and Hall, New York
Mraz W, Jatzkewitz H (1974) Cerebroside sulphatase activity of arylsulphatases from various invertebrates. Hoppe-Seyler's Z Physiol Chem 355:33–44
Murooka Y, Adachi T, Okamura H, Harada T (1977) Genetic control of arylsulfatase synthesis in Klebsiella aerogenes. J Bacteriol 130:74
Nelson MS, Scandalios JG (1977) Developmental expression and biochemical characterization of catalases and aminopeptides of Nassarius obsoleta. J Exp Zool 199:257–268
Norris E, Morrill JB (1964) An electrophoretic analysis of hydrolytic enzymes in adult organs and developing embryos of Limnaea palustris. Acta Embyrol Morphol Exp 7:29–41
Owen G (1966) Digestion. In: Wilbur KM, Yonge CM (eds) Physiology of Mollusca, vol 2, Academic Press, New York pp 53–96
Ragan M, Jensen A (1979) Widespread distribution of sulfated polyphenols in brown algae. Phytochemistry 18:261–262
Rapraeger AC, Epel D (1980) The appearance of an extracellular sulfatase during sea urchin embryogenesis. J Cell Biol 87:135a
Rapraeger AC, Epel D (1981) The appearance of an extracellular arylsulfatase during morphogenesis of the sea urchin Strongylocentrotus purpuratus. Dev Biol 88:269–278
Raven CP (1972) Chemical embryology of mollusca In: Florkin M, Scheer BT (eds) Chemical zoology: Mollusca, Vol 7, Academic Press, New York pp 155–180
Rees DA (1969) Structure, conformation and mechanism in the formation of polysaccharide gels and networks. Adv Carbohydr Chem Biochem 24:267–332
Sanguini LC, Pedersoli A, Dubois G, Masson M, Davolio E, Volpi N, Bolognani L (1984) Shift of optimal pH in arylsulphatase from Mollusca depending on temperature. Comp Biochem Physiol 78B:533–537
Sasaki H, Akasaka K, Shimada H, Shiroya T (1987a) Purification and characterization of arylsulfatase from sea urchin (Hemicentrotus pulcherrimus) embryos. Comp Biochem Physiol 88B:147–152
Sasaki H, Akasaka K, Shiroya T, Shimada H (1987b) Developmental timing of synthesis and translation of arylsulfatase mRNA in sea urchin embryo. Dev Growth Differ 29:317–322
Shimony T, Nigrelli RF (1972) Studies on arylsulphatases in the barnacle Balanus eburneus. Marine Biol 14:349–358
Suzuki S, Takahashi N, Egami F (1957) Enzymic transsulfation from a phenol to carbohydrates. Biochim Biophys Acta 24:444–445
Suzuki S, Saito H, Yamagata T, Anno K, Seno N, Kawai Y, Furuhashi (1968) Formation of three types of disulfated disaccharides from chondroitan sulfates by chondroitinase digestion. J Biol Chem 243:1543–1560
Takahashi N, Egami F (1961) Hydrolysis of polysaccharide sulphate esters by a sulphatase preparation from Charonia lampas. Biochem J 80:384–386
Towbin H, Staehelin T, Gordon J (1979) Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: Procedure and some applications. Proc Natl Acad Sci USA 76:4350–4354
Trapido-Rosenthal HG, Morse DE (1986a) Availability of chemosensory receptors is down-regulated by habituation of larvae to a morphogenetic signal. Proc Nat Acad Sci 83:7658–7662
Trapido-Rosenthal HG, Morse DE (1986b) Regulation of receptor-mediated-settlement and metamorphosis in larvae of a gastropod mollusc (Haliotis rufescens). Bull Mar Sci 39:383–392
Webb EC, Morrow PFW (1959) The activation of an arylsulphatase from ox liver by chloride and other anions. Biochem J 73:7–12
Weinstein B, Rold T, Harrell C, Burns N, Waaland J (1975) Reexamination of the bromophenols in the red alga, Rhodomela larix. Phytochem 14:2667–2679
Yamagata T, Kawamura Y, Suzuki S (1966) Substrate specificity of two separate chondrosulfatases in Proteus vulgaris. Biochim Biophys Acta 115:250–261
Yamagata T, Saito H, Habachi O, Suzuki S (1968) Purification and properties of bacterial chondroitinases and chondrosulfatases. J Biol Chem 243:1523–1535
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Spaulding, D.C., Morse, D.E. Purification and characterization of sulfatases from Haliotis rufescens: evidence for changes in synthesis and heterogeneity during development. J Comp Physiol B 161, 498–515 (1991). https://doi.org/10.1007/BF00257905
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00257905