Summary
The pineal complex of Lampetra fluviatilis, Anguilla anguilla and Salmo gairdneri was studied by means of the indirect immunohistochemical antiopsin reaction.
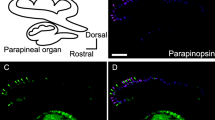
Opsin-immunoreactive material was demonstrated in the outer segments of the photoreceptor cells in the pineal organ of all three species investigated. In the lamprey, the opsin-positive outer segments were located in the lumen of the pineal vesicle and atrium. In the two teleost species, the immunoreactive outer segments were observed in abundance in the pineal end-vesicle and stalk. These structures were found to accumulate in the prominent initial portion of the pineal stalk of the eel. In the rainbow trout, immunoreactive outer segments occurred in the wide orifice of the pineal recess at the roof of the third ventricle.
In addition, outer segments of photoreceptor cells of the parapineal organ (“parapinealocytes”) displayed opsin immunoreactivity. In the lamprey, opsin immunoreactivity was restricted to the central portion of the ventral parapineal retina, while the parapinealocytes in the lateral portions did not bind the antibody. In the two teleosts, immunoreactive outer segments displayed a scattered pattern.
These immunocytochemical results provide direct evidence that the photosensitivity of the pineal demonstrated electrophysiologically in lampreys and teleosts (cf. Dodt 1973) is based on an opsin-containing photopigment. The presence of opsin in cells of the parapineal organ strengthens the view that also this organ may be capable of direct light perception. In the lamprey, the exclusive opsin immunoreactivity of a circumscribed group of parapineal cells suggests the existence of two types of parapinealocytes. The significance of opsin-containing photoreceptor outer segments occurring in the most proximal portion of the teleost pineal stalk is discussed, especially with regard to the interpretation of results obtained from pinealectomy experiments.
Similar content being viewed by others
References
Ali MA, Wagner HJ (1975) Visual pigments: physiology and ecology. In: Ali MA (ed) Vision in fishes. Plenum Publ. Corp., New York, pp 481–516
Bargmann W (1943) Die Epiphysis cerebri. In: Möllendorff W (ed) Handbuch der mikroskopischen Anatomie des Menschen, VI/1. Springer, Berlin pp 309–505
Basinger SF, Hoffman RT (1982) Rhodopsin biosynthesis in isolated retinas: Methods in Enzymology 81:772–782
Bertolini B, Manglia F (1966) Osservazioni sulla ultrastructura dell' occhio pineale della lampedra. Rend Acc Lincei 41:147–153
Blaustein DI, Dewey NM (1979) Localization of rhodopsin in isolated osmotically intact rod outer segment discs. J Histochem Cytochem 27:788–793
Bok D (1982) Renewal of photoreceptor cells. Methods in Enzymology 81:763–772
Breucker H, Horstmann E (1965) Elektronenmikroskopische Untersuchungen am Pinealorgan der Regenbogenforelle (Salmo irideus). Progr Brain Res 10:259–269
Cole WC, Youson JH (1982) Morphology of the pineal complex of the anadromous sea lamprey, Petromyzon marinus L. Am J Anat 165:131–163
Collin JP (1969a) Cellules ganglionnaires et tractus de l'organe pinéal de Lampetra planeri. J Neuro-Visc Relat 31: 308–333
Collin JP (1969b) Contribution à l'étude de l'organe pinéal. De l'épiphyse sensorielle à la glande pinéale: modalités de transformation et implications fonctionelles. Ann Stn Biol (Besse-en-Chandesse/France) Suppl 1:1–359
Collin JP, Oksche A (1981) Structural and functional relationships in the nonmammalian pineal organ. In: Reiter RJ (ed) The pineal gland: Anatomy and Biochemistry, Vol I, CRC Press, Boca Raton, Florida, pp 27–67
Converse CA, Papermaster DS (1975) Membrane protein analysis by two-dimensional immunoelectrophoresis. Science (Wash DC) 189:469–472
Dartnall HJA, Lander MR, Munz FW (1961) Periodic changes in the visual pigment of a fish. Progr Photobiol, Proc 3rd Int Photobiol Congr, Elsevier, Amsterdam, pp 203–213
Dodt E (1963) Photosensitivity of the pineal organ in the teleost, Salmo irideus (Gibbons). Experientia 19:642
Dodt E (1973) The parietal eye (pineal and parapineal organs) of lower vertebrates. In: Jung R (ed) Handbook of physiology VII/3B, Springer, Berlin Heidelberg New York, pp 113–140
Eakin R (1962) Lines of evolution of photoreceptors. J Gen Physiol 46:357 A-367 A
Eddy JMP, Strahan R (1968) The role of the pineal complex in the pigmentary effector system of the lampreys, Mordacia mordax (Richardson) and Geotria australis Gray. Gen Comp Endocrinol 11:528–534
Fenwick JC (1969) The pineal organ. In: Hoar WS, Randall DJ (eds) Fish physiology Vol 4, Academic Press, New York, pp 91–108
Fenwick JC (1970) Effects of pinealectomy and bilateral enucleation on the phototactic response and the conditioned response to light of the goldfish, Carassius auratus. Can J Zool 48:175–182
Gordon J, Shapley RM, Kaplan E (1978) The eel retina: Receptor classes and spectral mechanisms. J Gen Physiol 71:123–138
Hafeez MA, Zerihun L (1974) Studies on central projections of the pineal nerve tract in rainbow trout, Salmo gairdneri Richardson, using cobalt chloride iontophoresis. Cell Tissue Res 154:485–510
Hamasaki DI, Streck P (1971) Properties of the epiphysis cerebri of the small-spotted dogfish, Scyliorhinus canicula L. Vision Res 11:189–198
Hanyu I, Niwa H (1970) Pineal photosensitivity in three teleosts, Salmo irideus, Plecoglossus altivelis and Mugil cephalus. Rev Can Biol 29:133–140
Hanyu I, Niwa H, Tamura T (1978) Salient features of photosensory function of the teleostean pineal organ. Comp Biochem Physiol 61 A:49–54
Hárosi FI (1982) Polarized microspectrophotometry for pigment orientation and concentration. Methods in Enzymology 81:642–647
Hartwig HG (1975) Neurobiologische Studien an photoneuroendokrinen Systemen. Habil Thesis, Justus Liebig Univ. Gießen
Hartwig HG (1982) Synchrone Erneuerung der Außengliedstrukturen in der Epiphysis cerebri bei Rana esculenta. 3. Arbeitstagung der Anat. Ges., Würzburg 6.–8. Okt. 1982, Anat Anz Suppl (im Druck)
Hartwig HG, Baumann C (1974) Evidence for photosensitive pigments in the pineal complex of the frog. Vision Res 14:597–598
Hartwig HG, Oksche A (1982) Neurobiological aspects of extraretinal photoreceptive systems: structure and function. Experientia 38:991–996
Hartwig HG, Pfautsch M (1973) Rasterelektronenmikroskopische Beobachtungen an pinealen Sinneszellen der Forelle, Salmo gairdneri (Teleostei). Z Zellforsch 138:585–589
Hartwig HG, Van Veen Th (1979) Spectral characteristics of visible radiation penetrating into the brain and stimulating extraretinal photoreceptors. J Comp Physiol 130:277–282
Heller J (1969) Comparative study of a membrane protein. Characterization of bovine, rat and frog visual pigments. Biochemistry 8:675–678
Hill C (1894) The epiphysis of teleosts and Amia. J Morphol 9:237–268
Joss JMP (1973) Pineal-gonad relationship in the lamprey Lampetra fluviatilis. Gen Comp Endocrinol 21:118–122
Kavaliers M (1979) The pineal organ and circadian organization of teleost fish. Rev Can Biol 38:281–292
Korf HW (1974) Acetylcholinesterase-positive neurons in the pineal and parapineal organs of the rainbow trout, Salmo gairdneri (With special reference to the pineal tract). Cell Tissue Res 155:475–489
Korf HW, Wagner U (1981) Nervous connections of the parietal eye in adult Lacerta s. sicula Rafinesque as demonstrated by anterograde and retrograde transport of horseradish peroxidase. Cell Tissue Res 219:567–583
Korf HW, Liesner R, Meissl H, Kirk A (1981) Pineal complex of the clawed toad, Xenopus laevis Daud.: Structure and function. Cell Tissue Res 216:113–130
Korf HW, Zimmermann NH, Oksche A (1982) Intrinsic neurons and neural connections of the pineal organ of the house sparrow, Passer domesticus, as revealed by anterograde and retrograde transport of horseradish peroxidase. Cell Tissue Res 222:243–260
La Motte I de (1963) Untersuchungen zur vergleichenden Physiologie der Lichtempfindlichkeit geblendeter Fische. Naturwissenschaften 50/9:363
Liebman PA (1972) Microspectrophotometry of photoreceptors. In: Dartnall HJA (ed) The handbook of sensory physiology VII/1, Springer, Berlin Heidelberg New York, pp 481–528
Loew ER, Dartnall HJA (1976) Vitamin A1/A2-based visual pigment mixtures in cone of the rudd. Vision Res 16:891–896
Loew ER, Lythgoe JN (1978) The ecology of cone pigments in teleost fishes. Vision Res 18:715–722
Matsuura T, Herwig HJ (1981) Histochemical and ultrastructural study of the nervous elements in the pineal organ of the eel, Anguilla anguilla. Cell Tissue Res 216:545–557
Mayor HD, Hampton JC, Rosario B (1961) A simple method for removing the resin from epoxy-embedded tissue. J Biophys Biochem Cytol 9:909–910
McNulty JA (1981) Synaptic ribbons in the pineal organ of the goldfish: circadian rhythmicity and the effect of constant light and constant darkness. Cell Tissue Res 215:491–497
Meiniel A (1969) Cellules de type photorécepteur dans la rétine dorsale de l'organe parapinéal d'ammocète de Lampetra planeri. C R Acad Sci (Paris) Sér D 268:2265–2268
Meiniel A (1971) Etude cytophysiologique de l'organe parapinéal de Lampetra planeri. J NeuroVisc Rel 32:157–199
Meiniel A (1980) Ultrastructure of serotonin-containing cells in the pineal organ of Lampetra planeri (Petromyzontidae). A second sensory cell line from photoreceptor to pinealocyte. Cell Tissue Res 207:407–427
Meiniel A (1981) New aspects of the phylogenetic evolution of sensory cell lines in the vertebrate pineal complex. Developments in Endocrinology 14:27–48
Meiniel A, Collin JP (1971) Le complexe pinéal de l'ammocète (Lampetra planeri, Bl.). Identification de ganglion sous-jacent à l'organe parapinéal et relations épithalamique des organes pinéal et parapinéal. Z Zellforsch 117:354–380
Meiniel A, Vivien-Roels B (1983) The presence of two populations of sensory-type cells in the pineal organ of the five-bearded rockling, Ciliata mustela L. (Pisces, Teleostei). Cell Tissue Res, in press
Meissl H, Dodt E (1981) Comparative physiology of pineal photoreceptor organs. Developments in Endocrinology 14:61–80
Morita Y (1966) Entladungsmuster pinealer Neurone der Regenbogenforelle (Salmo irideus) bei Belichtung des Zwischenhirns. Pflügers Arch ges Physiol 289:155–167
Morita Y (1975) Direct photosensory activity of the pineal. In: Knigge KM, Scott DE, Kobayashi H, Ishii S (eds) Brain-Endocrine-Interaction II. The ventricular system, Karger, Basel, pp 376–387
Morita Y, Dodt E (1971) Photosensory responses from the pineal eye of the lamprey (Petromyzon fluviatilis). Proc Int Physiol Sci 25th, Munich 9:405
Morita Y, Dodt E (1973) Slow photic responses of the isolated pineal organ of lamprey. Nova Acta Leopoldina 38:331–339
Nilsson SEG, Crescitelli F (1970) A correlation of ultrastructure and function in the developing retina of the frog tadpole. J Ultrastruct Res 30:87–102
Oksche A (1965) Survey of the development and comparative morphology of the pineal organ. Progr Brain Res 10:3–29
Oksche A, Hartwig HG (1975) Photoneuroendocrine systems and the third ventricle. In: Knigge KM, Scott DE, Kobayashi H, Ishii S (eds) Brain-endocrine interaction II. The ventricular system, Karger, Basel, pp 40–53
Oksche A, Hartwig HG (1979) Pineal sense organs — components of photoneuroendocrine systems. Progr Brain Res 52:113–130
Oksche A, Vaupel-von Harnack M (1965) Vergleichende elektronenmikroskopische Studien am Pinealorgan. Progr Brain Res 10:237–258
Omura Y (1979) Light and electron microscopic studies on the pineal tract of rainbow trout, Salmo gairdneri. Rev Can Biol 38:105–118
Omura Y, Ali MA (1980) Responses of pineal photoreceptors in the brook and rainbow trout. Cell Tissue Res 208:111–122
Papermaster DS, Converse CA, Siu J (1975) Membrane biosynthesis in the frog retina: opsin transport in the photoreceptor cell. Biochemistry 14:1343–1352
Papermaster DS, Converse CA, Zorn MA (1976) Biosynthesis and immunocytochemical characterization of a large protein in frog and cattle rod outer segment membranes. Exp Eye Res 23:105–116
Papermaster DS, Schneider BG, Zorn MA (1978) Immunocytochemical localization of opsin in outer segments and Golgi zones of frog photoreceptor cells. An electron microscope analysis of cross-linked albumin-embedded retinas. J Cell Biol 77:196–120
Pu GA, Dowling JE (1981) Anatomical and physiological characteristics of pineal photoreceptor cell in the larval lamprey, Petromyzon marinus. J Neurophysiol 46:1018–1038
Röhlich P (1976) Photoreceptor membrane carbohydrate on the intradiscal surface of retinal rod disks. Nature 263:789–791
Rüdeberg C (1969) Structure of the parapineal organ of the adult rainbow trout, Salmo gairdneri Richardson. Z Zellforsch 93:282–304
Rüdeberg C (1971) Structure of the pineal organ of Anguilla anguilla L. and Lebistes reticulatus Peters (Teleostei). Z Zellforsch 122:227–243
Schwanzara SA (1967) The visual pigments of freshwater fishes. Vision Res 7:121–148
Stell WK, Hárosi FI (1976) Cone structure and visual pigment content in the retina of the goldfish. Vision Res 16:647–658
Studnička FK (1893) Sur les organes pariétaux de Petromyzon planeri. Sitzung Gesellschaft Wissenschaft (Prague): 1–50
Studnička FK (1905) Die Parietalorgane. In: Oppel A (ed) Lehrbuch der vergleichenden mikroskopischen Anatomie der Wirbeltiere. Vol 5 A, Fischer, Jena, pp 1–254
Tabata M, Tamura T, Niwa H(1975) Origin of the slow potential in the pineal organ of the rainbow trout. Vision Res 15:737–740
Tilney F, Warren LF (1919) The morphology and evolutional significance of the pineal body. Am Anat Memoirs 9:1–257
Tretjakoff D (1915) Die Parietalorgane von Petromyzon fluviatilis. Z wiss Zool 113:1–112
Tsin ATC, Liebman PA, Beatty DD, Drzymaka R (1981) Rod and cone visual pigment in the goldfish. Vision Res 21:943–946
Ueck M (1979) Innervation of the vertebrate pineal. Progr Brain Res 52:45–88
Ueck M (1981) Variation in structure and function of the pineal systems. Developments in Endocrinology 14:151–168
Van Veen Th (1981) A study on the basis for zeitgeber entrainment. With special reference to extraretinal photoreception in the eel. Dr phil thesis, Mat Nat Fac Univ Lund
Van Veen Th (1982) The pineal and parapineal organs of the elver (Anguilla anguilla L.). Cell Tissue Res 222:433–444
Van Veen Th, Andersson H (1982) Threshold for synchronization of locomotor activity to visible radiation in the eel Anguilla anguilla. OIKOS 38:21–26
Van Veen Th, Hartwig HG, Müller K (1976) Light-dependent motor activity and photonegative behavior in the eel (Anguilla anguilla L.). Evidence for extraretinal and extrapineal photoreception. J Comp Physiol 111:209–219
Vigh B, Vigh-Teichmann I (1981) Light- and electron-microscopic demonstration of immunoreactive opsin in the pinealocytes of various vertebrates. Cell Tissue Res 221:451–463
Vigh B, Vigh-Teichmann I, Aros B (1975) Comparative ultrastructure of cerebrospinal fluid-contacting neurons and pinealocytes. Cell Tissue Res 158:409–424
Vigh B, Vigh-Teichmann I, Röhlich P, Aros B (1982) Immunoreactive opsin in the pineal organ of reptiles and birds. Z mikr-anat Forsch 96:113–129
Vigh-Teichmann I (1981) The pineal eye of vertebrates. Acta Anat 111:155
Vigh-Teichmann I, Röhlich P, Vigh B, Aros B (1980a) Comparison of the pineal complex, retina and cerebrospinal fluid-contacting neurons by immunocytochemical antirhodopsin reaction. Z mikr-anat Forsch 94:623–640
Vigh-Teichmann I, Vigh B, Röhlich P, Olsson R (1980b) Phylogenetic aspects of the sensory neurons of the wall of the diencephalon. In: Spatz M, Mrsulja BB, Rakic LjM, Lust WD (eds) Circulatory and developmental aspects of brain metabolism, Plenum Press, New York, pp 415–428
Vigh-Teichmann I, Korf HW, Oksche A, Vigh B (1982a) Opsin-immunoreactive outer segments and acetylcholinesterase positive neurons in the pineal complex of Phoxinus phoxinus (Teleostei, Cyprinidae). Cell Tissue Res 227:351–370
Vigh-Teichmann I, Vigh B, Manzano e Silva MJ, Aros B (1983) The pineal organ of Raja clavata: Opsin immunoreactivity and ultrastructure. Cell Tissue Res 228:139–148
Vigh-Teichmann I, Korf HW, Nürnberger F, Oksche A, Vigh B (1982b) Vergleichende immuncytochemische Studien an pinealen Photorezeptoren und Liquorkontaktneuronen. Verh Anat Ges 77, im Druck
Vivien-Roels B (1981) Pineal control of reproduction in non-mammalian vertebrates. Developments in Endocrinology 14:315–334
Vollrath L (1981) The pineal organ. In: Oksche A, Vollrath L (eds) Handbuch der mikroskopischen Anatomie des Menschen VI/7, Springer, Berlin Heidelberg New York
Yamada E (1982) Morphology of vertebrate photoreceptors. Methods in Enzymology 81:3–17
Young JZ (1935) The photoreceptors of lampreys. II. The function of the pineal complex. J Exp Biol 12:254–270
Author information
Authors and Affiliations
Additional information
Dedicated to Professor E. Dodt, Max Planck Institute for Physiological and Clinical Research, Bad Nauheim, FRG, on the occasion of his 60th birthday
This investigation was supported by grants from the Deutsche Forschungsgemeinschaft to A.O. (Ok 1/25: Mechanismen biologischer Uhren) and to H.W.K. (Ko 758/1; 758/2-2), and from the Swedish Natural Research Sciences Council to R.O. (Nr. 2124)
On leave from the 2nd Department of Anatomy, Semmelweis OTE, Budapest, Hungary
Rights and permissions
About this article
Cite this article
Vigh-Teichmann, I., Korf, H.W., Nürnberger, F. et al. Opsin-immunoreactive outer segments in the pineal and parapineal organs of the lamprey (Lampetra fluviatilis), the eel (Anguilla anguilla), and the rainbow trout (Salmo gairdneri). Cell Tissue Res. 230, 289–307 (1983). https://doi.org/10.1007/BF00213806
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00213806