Abstract
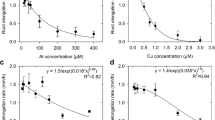
The effects of aluminum on the concentration-dependent kinetics of Ca2+ uptake were studied in two winter wheat (Triticum aestivum L.) cultivars, Al-tolerant Atlas 66 and Al-sensitive Scout 66. Seedlings were grown in 100 μM CaCl2 solution (pH 4.5) for 3 d. Subsequently, net Ca2+ fluxes in intact roots were measured using a highly sensitive technique, employing a vibrating Ca2+-selective microelectrode. The kinetics of Ca2+ uptake into cells of the root apex, for external Ca2+ concentrations from 20 to 300 μM, were found to be quite similar for both cultivars in the absence of external Al; Ca2+ transport could be described by Michaelis-Menten kinetics. When roots were exposed to solutions containing levels of Al that were toxic to Al-sensitive Scout 66 but not to Atlas 66 (5 to 20 μM total Al), a strong correlation was observed between Al toxicity and Al-induced inhibition of Ca2+ absorption by root apices. For Scout 66, exposure to Al immediately and dramatically inhibited Ca2+ uptake over the entire Ca2+ concentration range used for these experiments. Kinetic analyses of the Al-Ca interactions in Scout 66 roots were consistent with competitive inhibition of Ca2+ uptake by Al. For example, exposure of Scout 66 roots to increasing Al levels (from 0 to 10 μM) caused the K m for Ca2+ uptake to increase with each rise in Al concentration, from approx. 100 μM in the absence of Al to approx. 300 μM in the presence of 10 μM Al, while having no effect on the V max. The same Al exposures had little effect on the kinetics of Ca2+ uptake into roots of Atlas 66. The results of this study indicate that Al disruption of Ca2+ transport at the root apex may play an important role in the mechanisms of Al toxicity in Al-sensitive wheat cultivars, and that differential Al tolerance may be associated with the ability of Ca2+-transport systems in cells of the root apex to resist disruption by potentially toxic levels of Al in the soil solution.
Similar content being viewed by others
Referencess
Bennet, R.J., Breen, C.M., Fey, M.V. (1985) Aluminum uptake sites in the primary roots of Zea may L. S. Afr. J. Plant Soil 2, 1–7
Deleers, M. (1986) Cationic atmosphere and cation competition binding at negatively charged membranes: Pathological implications of aluminum. Res. Commun. Chem. Pathol. Pharmacol. 49, 277–294
Foy, C.D. (1988) Plant adaptation to acid, aluminum-toxic soils. Commun. Soil Sci. Plant Anal. 19, 959–987
Foy, C.D., Chaney, R.L., White, M.C. (1978) The physiology of metal toxicity in plants. Annu. Rev. Plant Physiol. 29, 511–566
Haug, A.R. (1984) Molecular aspects of aluminum toxicity. CRC Crit. Rev. Plant Sci. 1, 345–373
Haug, A.R., Caldwell, C.R. (1985) Aluminum toxicity in plants: the role of the root plasma membrane and calmodulin. In: Frontiers of membrane research in agriculture, pp. 359–382, St. John, J.B., Berlin, E., Jackson, P.C., eds. Rowman and Allanheld, Totowa
Hecht-Bucholz, C., Foy, C.D. (1981) Effect of aluminum toxicity on root morphology of barley. Plant Soil 63, 93–95
Hille, B. (1992) Ionic channels of excitable membranes. 2nd Edition, Sinauer Associates, Inc., Sunderland, Mass., USA
Huang, J.W., Shaff, J.E., Grunes, D.L., Kochian, L.V. (1992) Aluminum effects on calcium fluxes at the root apex of aluminumtolerant and aluminum-sensitive wheat cultivars. Plant Physiol. 98, 230–237
Jaffe, L.F., Levy, S. (1987) Calcium gradients measured with a vibrating calcium-selective electrode. Inst. Elect. Eng. Med. Biol. Soc. Conf. 9, 779–781
Kinraide, T.B. (1988) Proton extrusion by wheat root exhibiting severe aluminum toxicity symptoms. Plant Physiol. 88, 418–423
Kinraide, T.B., Ryan, P.R., Kochian, L.V. (1992) Interactive effects of Al3+, H+, and other cations on root elongation considered in terms of cell-surface electrical potential. Plant Physiol. 99, 1461–1468
Kochian, L.V., Shaff, J.E., Kühtreiber, W.M., Jaffe, I.F., Lucas, W.J. (1992) The use of an extracellular, ion-selective, vibrating-microelectrode system for the quantification of K+, H+, and Ca2+ fluxes in maize roots and maize suspension cells. Planta, in press
Kühtreiber, W.M., Jaffe, L.F. (1990) Detection of extracellular calcium gradients with a calcium-specific vibrating electrode. J. Cell Biol. 110, 1565–1573
Miyasaka, S.C., Kochian, L.V., Shaff, J.E., Foy, C.D. (1989) Mechanisms of aluminum tolerance in wheat. An investigation of genotypic differences in rhizosphere pH, K+, and H+ transport, and root-cell membrane potentials. Plant Physiol. 91, 1188–1196
Miyasaka, S.C., Buta, B.G., Howell, R.K., Foy, C.D. (1991) Mechanism of aluminum tolerance in snapbeans. Root exudation of citric acid. Plant Physiol. 96, 737–743
Schroeder, J.I. (1988) K+ transport properties of K+ channels in the plasma membrane of Vicia faba guard cells. J. Gen. Physiol. 92, 667–683
Segel, I.H. (1976) Biochemical calculations: How to solve mathematical problems in general biochemistry, 2nd Edn., John Wiley & Sons, Inc., USA
Suhayda, C.G., Haug, A. (1985) Citrate chelation as a potential mechanism against aluminum toxicity in cells: the role of calmodulin. Can. J. Biochem. Cell Biol. 63, 1167–1175
Taylor, G.J. (1988a) The physiology of aluminum phytotoxicity. In: Metal ions in biological systems, vol. 24: Aluminum and its role in biology, pp. 123–163, Sigel, H., ed. Marcel-Dekker, New York
Taylor, G.J. (1988b) The physiology of aluminum tolerance. In: Metal ions in biological systems, vol. 24: Aluminum and its role in biology, pp. 165–198, Sigel, H., ed. Marcel-Dekker, New York
Vierstra, R., Haug, A. (1978) The effect of Al3+ on the physical properties of membrane lipids in Thermoplasma acidophilum. Biochem. Biophys. Res. Commun. 84, 138–143
Zhao, X.J., Sucoff, E., Stadelmann, E.F. (1987) Al3+ and Ca2+ alteration of membrane permeability of Quercus rubra root cortex cells. Plant Physiol. 83, 159–162
Author information
Authors and Affiliations
Additional information
We would like to thank Dr. Lionel F. Jaffe, Director of the National Vibrating Probe Facility, Marine Biological Laboratory, Woods Hole, Mass., USA, for making his calcium-selective vibrating-mi-croelectrode system available for a portion of this work. The research presented here was supported in part by USDA/NRI Competitive Grant number 91-37100-6630 to Leon Kochian. Contribution from the USDA-ARS, U.S. Plant, Soil and Nutrition Laboratory, Cornell University, Ithaca, N.Y. This research was part of the program of the Center for Root-Soil Research, Cornell University, Ithaca, N.Y. Department of Soil, Crop and Atmosphere Science, paper No. 1741.
Rights and permissions
About this article
Cite this article
Huang, J.W., Grunes, D.L. & Kochian, L.V. Aluminum effects on the kinetics of calcium uptake into cells of the wheat root apex. Planta 188, 414–421 (1992). https://doi.org/10.1007/BF00192809
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00192809