Summary
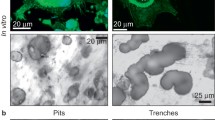
This study examined the relationship between the number of nuclei in an osteoclast and its resorptive efficiency, as demonstrated by the size of the pit it can make in a mineralized tissue in 24 h in vitro. Osteoclasts released mechanically from prehatch chick long bones were cultured on dentine slices or on plastic dishes for periods of 6 or 24 h. The frequency distribution of the multinucleate tartrate-resistant acid phosphatase (TRAP)-positive cells with different numbers of nuclei was determined: the mean number of nuclei per cell was 6.92, with a mode of 4. 47% had 5 or fewer nuclei and only 11% more than 10 nuclei. The pits associated with 292 osteoclasts with known numbers of nuclei were measured using a confocal laser light microscope (Lasertec) and dedicated image analysis system, and depths, plan areas and volumes determined. There was a positive correlation between the number of nuclei per osteoclast and the volume of the pit made, but a trend for the volume resorbed per nucleus to decrease with increase in the number of nuclei per osteoclast.
Similar content being viewed by others
References
Addison WC (1978) The distribution of nuclei in human odontoc-lasts in whole cell preparations. Arch Oral Biol 23:1167–1171
Addision WC (1980) The effect of parathyroid hormone on the numbers of nuclei in feline osteoclasts in vivo. J Anat 130:479–486
Amano S, Hanazawa S, Kawata Y, Ohta K, Kitami H, Kitano S (1992) An assay system utilizing devitalized bone for assessment of differentiation of osteoclast precursors. J Bone Min Res 7:321–328
Athanasou NA, Puddle B, Quinn J, Woods CG (1991) Use of monoclonal antibodies to recognise osteoclasts in routinely processed bone biopsy specimens. J Clin Pathol 44:664–666
Baron R, Neff L, Louvard D. Courtoy PJ (1985) Cell-mediated extracellular acidification and bone resorption: evidence for a low pH in resorbing lacunae and localization of a 100kD lyso-somal membrane protein at the osteoclast ruffled border. J Cell Biol 101:2210–2222
Basle MF, Mazaud P, Malkani K, Chretien MF, Moreau MF, Rebel A (1988) Isolation of osteoclasts from Pagetic bone tissue. Morphometry and cytochemistry on isolated cells. Bone 9:1–6
Boyde A, Jones SJ (1979) Estimation of the size of resorption lacunae in mammalian calcified tissues using SEM stereophoto-grammetry. Scanning Electron Microsc/1979/2:393–402
Boyde A, Jones SJ (1987) Early scanning electron microscopic studies of hard tissue resorption: their relation to current concepts reviewed. Scanning Microsc 1:369–381
Boyde A, Jones SJ (1991) Pitfalls in pit measurement. Calcif Tissue Int 49:65–70
Boyde A, Jones SJ (1992) Really close up! Surveying surfaces at submicrometre resolution: the measurement of osteoclastic resorption lacunae. Photogramm Rec 14:59–84
Boyde A, Ali NN, Jones SJ (1983) Computer-aided measurement of resorptive activity of isolated osteoclasts. Proc Microsc Soc 18:357
Delaisse J-M, Boyde A, Maconnachie E, Ali NN, Sear CHJ, Eeck-hout Y, Vaes G, Jones SJ (1987) The effect of inhibitors of cysteine-proteinases and collagenase on the resorptive activity of isolated osteoclasts. Bone 8:303–313
Groessner-Schreiber B, Krukowski M, Hertweck D, Osdoby P (1991) Osteoclast formation is related to bone matrix age. Calcif Tissue Int 48:335–340
Hattersley G, Chambers TJ (1989) Generation of osteoclastic function in mouse bone marrow cultures: multinuclearity and tar-trate-resistant acid phosphatase are unreliable markers for osteoclastic differentiation. Endocrinology 124:1689–1696
Hefley TJ, Stern PH (1982) Isolation of osteoclasts from fetal rat long bones. Calcif Tissue Int 34:480–487
Holtrop ME, King GJ, Cox KA, Reit B (1979) Time-related changes in the ultrastructure of osteoclasts after injection of parathyroid hormone in young rats. Calcif Tissue Int 27:129–135
Jones SJ, Boyde A (1988) The resorption of dentine and cementum in vivo and in vitro. In: Davidovitch Z (ed) The biological mechanisms of tooth eruption and root resorption. EBSCO Media, Birmingham, Al 35233, pp 335–354
Jones SJ, Boyde A, Ali NN, Maconnachie E (1986) Variation in the sizes of resorption lacunae made in vitro. Scanning Electron Microsc 1986IV: 1571–1580
Kanehisa J, Heersche JN (1988) Osteoclastic bone resorption: in vitro analysis of the rate of resorption and migration of individual osteoclasts. Bone 9:73–79
Katoh Y, Tsuji H, Matsui H, Maruta K, Morita Y (1991) Effects of ethane-1-hydroxy-1, 1-diphosphonate on cell differentiation, and proteoglycan and calcium metabolism, in the proximal tibia of young rats. Bone 12:59–65
Kember NF (1960) Cell division in endochondral osteogenesis in young rats. J Cell Biol 14:357–370
Kukita A, Chenu C, McManus LM, Mundy GR, Roodman GD (1990) Atypical multinucleated cells form in long-term marrow coltures from patients with Paget's disease. J Clin Invest 85:1280–1286
Raff MC (1992) Social controls on cell survival and cell death. Nature 356:397–400
Ries WL, Gong JK (1982) A comparative study of osteoclasts: in situ versus smear specimens. Anat Rec 203:221–232
Ries WL, Gong JK, Gunsolley JC (1987) The distribution and kinetics of nuclei in rat osteoclasts. Cell Tissue Kinet 20:1–14
Shapiro F, Key LL, Anast C (1988) Variable osteoclast appearance in human infantile osteopetrosis. Calcif Tissue Int 43:67–76
Taylor ML, Maconnachie E, Frank K, Boyde A, Jones SJ (1990) The effect of fluoride on the resorption of dentine by osteoclasts in vitro. J Bone Min Res 5 [Suppl 1]:S121-S130
Umita S, Nicholson GC, Rowe DJ, Kent GN, Martin TJ (1991) Biphasic effect of calcitonin on tartrate-resistent acid phospha-tase activity in isolated rat osteoclasts. J Bone Min Res 6:591–597
Vignery A, Wang F, Qian HY, Benz EJ, Gilmore-Herbert M (1991) Detection of the Na+-K+- ATPase alpha 3-isoform in multinucleated macrophages. Am J Physiol 260: F704–709
Young RW (1962) Cell proliferation and specialization during endochondral ossification. A study of cell division in rat bones by the method of tritiated autoradiography. J Bone Joint Surg 428:824–839
Zambonin Zallone A, Teti A (1981) The osteoclasts of hen medullary bone under hypocalcaemic conditions. Anat Embryol 162:379–392
Zheng MH, Papadimitriou JM, Nicholson GC (1991) RNA synthesis in isolated rat osteoclasts: inhibitory effect of calcitonin. Bone 12:317–322
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Piper, K., Boyde, A. & Jones, S.J. The relationship between the number of nuclei of an osteoclast and its resorptive capability in vitro. Anat Embryol 186, 291–299 (1992). https://doi.org/10.1007/BF00185977
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00185977