Summary
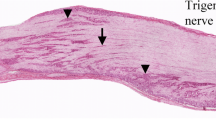
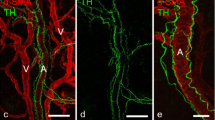
Healing of an experimental bony defect in the rat's tibia was studied with an immunofluorescent technique to clarify when and where substance P (SP) and calcitonin gene-related peptide (CGRP) would develop. The normal tibia showed a few SP- and CGRP-immunofluorescent nerve fibres. In the experimental tibia, the number of these fibres increased on the 6th day after operation, reached a peak of proliferation on the 15th day and reverted to normal after the 24th day. The changes were associated with the development and decay of callus tissue suggesting that harmful stimuli from the injured site in a bone could be mediated by sensory nerves throughout the repair period. Most of the SP- and CGRP-immunofluorescence was seen near the vessels, frequently in the same nerve fibres. The SP- and CGRP-immunofluorescent nerves seemed to take part jointly in callus formation through the enhancement of local blood flow.
Résumé
Le procesus de guérison d'une perte de substance osseuse expérimentale a été étudié sur le tibia du rat par immunofluorescence afin de déterminer quand et où la substance P (SP) et la calcitonine peptide d'origine génique (CGRP) se développeraient. Le tibia normal ne montre qu'un petit nombre de fibres nerveuses immunofluorescentes SP et CGRP. Dans le tibia d'expérimentation, les fibres nerveuses immunofluorescentes SP et CGRP augmentent en nombre à partir du 6ème jour, atteignent leur maximum de prolifération le 15ème jour et reviennent à l'état normal après le 24ème jour post-opératoire. Ces modifications sont étroitement associées au développement et à la disparition du cal, suggérant ainsi que les stimuli nocifs provenant de la lésion osseuse pourraient être inactivés par les nerfs sensitifs durant la période de réparation. En outre, la plus grande partie de l'immunofluorescence SP et CGRP a été observée à proximité des vaisseaux, souvent dans les mêmes fibres nerveuses. Il semble que les nerfs immunofluorescents SP et CGRP participent conjointement à la formation du cal en augmentant la vascularisation locale.
Similar content being viewed by others
References
Aro H, Eerola E, Aho AJ (1985) Fracture healing in paraplegic rats. Acta Orthop Scand 56: 228–232
Bernard GW, Shih C (1990) The osteogenic stimulating effect of neuroactive calcitonin gene-related peptide. Peptides 11: 625–632
Bjurholm A (1991) Neuroendocrine peptides in bone. Int Orthop 15: 325–329
Bjurholm A, Kreicbergs A, Brodin E, Schultzberg M (1988) Substance P- and CGRP-immunoreactive nerves in bone. Peptides 9: 165–171
Bjurholm A, Kreicbergs A, Schultzberg M (1989) Fixation and demineralization of bone tissue for immunohistochemical staining of neuropeptides. Calcif Tissue Int 45: 227–231
Brain SD, Williams TJ, Tippins JR, Morris HR, MacIntyre I (1985) Calcitonin gene-related peptide is a potent vasodilator. Nature 313: 54–56
Brain SD, Williams TJ (1988) Substance P regulates the vasodilator activity of calcitonin gene-related peptide. Nature 335: 73–75
Crossmann DC, Larkin SW, Dashwood MR, Davies GJ, Yacoub M, Maseri A (1991) Responses of atherosclerotic human coronary arteries in vivo to the endothelium-dependent vasodilator substance P. Circulation 84: 2001–2010
D'Souza SM, MacIntyre I, Girgis SI, Mundy GR (1986) Human synthetic calcitonin gene-related peptide inhibits bone resorption in vitro. Endocrinology 119: 58–61
Frymoyer JW, Pope MH (1977) Fracture healing in the sciatically denervated rat. J Trauma 17: 355–361
Grönblad M, Liesi P, Korkala O, Karaharju E, Polak J (1984) Innervation of human bone periosteum by peptidergic nerves. Anat Rec 209: 297–299
Grönblad M, Weinstein JN, Santavirta S (1991) Immunohistochemical observations on spinal tissue innervation. Acta Orthop Scand 62: 614–622
Hill EL, Elde R (1991) Distribution of CGRP-, VIP-, DβH-, SP-, and NPY-immunoreactive nerves in the periosteum of the rat. Cell Tissue Res 264: 469–480
Hill EL, Elde R (1988) Calcitonin gene-related peptide-immunoreactive nerve fibers in mandibular periosteum of rat. Evidence for primary afferent origin. Neurosci Lett 85: 172–178
Hohmann EL, Elde RP, Rysavy JA, Einzig S, Gebhard RL (1986) Innervation of periosteum and bone by sympathetic vasoactive intestinal peptide-containing nerve fibers. Science 232: 868–871
Hohmann EL, Levine L, Tashjian AH Jr (1983) Vasoactive intestinal peptide stimulates bone resorption via a cyclic adenosine 3′,5′-monophosphate-dependent mechanism. Endocrinology 112: 1233–1239
Hökfelt T, Holets VR, Staines W, Melander T, Schalling M, Schultzberg M, Freedmann J, Björklund H, Olson L, Lindh B, Elfvin L-G, Lundberg JM, Lindgren J, Samuelsson B, Pernow B, Terenius L, Post C, Everitt B, Goldstein M (1987) Coexistence of neuronal messengers. An overview. Prog Brain Res 68: 33–70
Kuraishi Y, Hirota N, Sato Y, Hino Y, Satou M, Takagi H (1985) Evidence that substance P and somatostatin transmit separate information related to pain in the spinal dorsal horn. Brain Res 325: 294–298
Lee Y, Kawai Y, Takami K, Hillyard CJ, Girgis S, MacIntyre I, Emson PC, Tohyama M (1985) Coexistence of calcitonin gene-related peptide and substance P-like peptide in single cells of the trigeminal ganglion of the rat. Immunohistochemical analysis. Brain Res 330: 194–196
Lee Y, Takami K, Kawai Y, Girgis S, Hillyard CJ, MacIntyre I, Emson PC, Tohyama M (1985) Distribution of calcitonin gene-related peptide in the rat peripheral nervous system with special reference to its coexistence with substance P. Neuroscience 15: 1227–1237
Matsuyama T, Wanaka A, Yoneda S, Kimura K, Kamada T, Girgis S, MacIntyre I, Emson CE, Tohyama M (1986) Two distinct calcitonin gene-related peptide containing peripheral nervous systems. Distribution and quantitative differences between the iris and cerebral artery with special reference to substance P. Brain Res 373: 205–212
Morton CR, Hutchison WD (1989) Release of sensory neuropeptides in the spinal cord. Studies with calcitonin gene-related peptide and galanin. Neuroscience 31: 807–815
Nakagawa Y, Shiosaka S, Emson PC, Tohyama M (1985) Distribution of neuropeptide Y in the forebrain and diencephalon. An immunohistochemical analysis. Brain Res 361: 52–60
Nakao Y, Hirasawa Y, Okada S, Ohta Y (1992) Callus formation and microvascular regeneration during the fracture healing process in the rat femur. J Jpn Orthop Assoc 66: 742–752 (in Japanese)
Rockwood CA Jr, Green DP, Bucholz RW (1975) Rockwood and Green's fractures in adults 3rd edn. Lippincott, Philadelphia, pp 186–203
Rusanen M, Korkala O, Grönblad M, Partanen S, Nederström A (1987) Evolution of substance P immuno-fluorescent nerves in callus tissue during fracture healing. J Trauma 27: 1340–1343
Tiseo PJ, Adler MW, Liu-Chen LY (1990) Different release of substance P and somatostatin in the rat spinal cord in response to noxious cold and heat. Effect of dynorphin A(1–17). J Pharmacol Exp Ther 252: 539–545
Urist MR, Maryland B, McLean FC (1941) Calcification in the callus in healing fractures in normal rats. J Bone Joint Surg 23: 1–5
Wiesenfeld-Hallin Z, Hökfelt T, Lundberg JM, Forssmann WG, Reinecke M, Tschopp FA, Fischer JA (1984) Immunoreactive calcitonin gene-related peptide and substance P coexist in sensory neurons to the spinal cord and interact in spinal behavioral responses of the rat. Neurosci Lett 52: 199–204
Zaidi M, Brain SD, Tippins JR, Marzo VD, Moonga BS, Chambers TJ, Morris HR, MacIntyre I (1990) Structureactivity relationship of human calcitonin gene-related peptide. Biochem J 269: 775–780
Zaidi M, Chambers TJ, Gaines Das RE, Morris HR, MacIntyre I (1987) A direct action of human calcitonin gene-related peptide on isolated osteoclasts. J Endocrinol 115: 511–518
Zaidi M, Fuller K, Bevis PJR, Gaines Das RE, Chambers TJ, MacIntyre I (1987) Calcitonin gene-related peptide inhibits osteoclastic bone resorption. A comparative study. Calcif Tissue Int 40: 149–154
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Aoki, M., Tamai, K. & Saotome, K. Substance P- and calcitonin gene-related peptide-immunofluorescent nerves in the repair of experimental bone defects. International Orthopaedics 18, 317–324 (1994). https://doi.org/10.1007/BF00180235
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00180235