Abstract
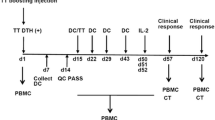
Dendritic cell-based active immunotherapies of cancer patients are aimed to provoke the proliferation and differentiation of tumor-specific CD4+ and CD8+ T-lymphocytes towards protective effector cells. Isolation and in vitro differentiation of circulating blood monocytes has been established a reasonable platform for adoptively transferred DC-based immunotherapies. In the present study the safety and tolerability of vaccination by autologous tumor cell lysates (oncolysate)- or carcinoembriogenic antigen (CEA)-loaded DCs in patients with colorectal cancer was investigated in a phase I-II trial. The study included 12 patients with histologically confirmed colorectal cancer (Dukes B2-C stages). Six of the patients received oncolysate-pulsed, whereas the other six received recombinant CEA-loaded autologous DCs. The potential of the tumor antigen-loaded DCs to provoke the patient’s immune system was studied both in vivo and in vitro. The clinical outcome of the therapy evaluated after 7 years revealed that none of the six patients treated with oncolysate-loaded DCs showed relapse of colorectal cancer, whereas three out of the six patients treated with CEA-loaded DCs died because of tumor relapse. Immunization with both the oncolysate- and the CEA-loaded autologous DCs induced measurable immune responses, which could be detected in vivo by cutaneous reactions and in vitro by lymphocyte proliferation assay. Our results show that vaccination by autologous DCs loaded with autologous oncolysates containing various tumor antigens represents a well tolerated therapeutic modality in patients with colorectal cancer without any detectable adverse effects. Demonstration of the efficacy of such therapy needs further studies with increased number of patients.


Similar content being viewed by others
Abbreviations
- 5-FU:
-
5-Fluorouracil
- AICD:
-
Activation-induced cell death
- AIMV:
-
Serum free therapeutic grade cell culture medium
- ALAT:
-
Alanine amino transferase
- AMA:
-
Antimitochondrial antibody
- ANA:
-
Antinuclear antibody
- APC:
-
Antigen presenting cell
- APTI:
-
Activated partial thromboplastin time
- ASAT:
-
Aspartate amino transferase
- CA19.9:
-
Cancer antigen 19.9
- CEA:
-
Carcinoembriogenic antigen
- CRC:
-
Colorectal carcinoma
- CTL:
-
Cytotoxic T lymphocyte
- DC:
-
Dendritic cell
- ECOG:
-
Eastern cooperative oncology group/patient performance status
- GM-CSF:
-
Granulocyte-monocyte colony-stimulating factor
- HBsAg:
-
Hepatitis B surface antigen
- HCV:
-
Hepatitis C virus
- HIV:
-
Human immunodeficiency virus
- HLA:
-
Human leukocyte antigen
- IFNγ:
-
Interferon gamma
- IL 12:
-
Interleukin 12
- IL 2:
-
Interleukin 2
- IL 1β:
-
Interleukin 1beta
- IL 6:
-
Interleukin 6
- INR:
-
Internation normalized ratio (coagulation)
- LDH:
-
Lactate dehydrogenase
- MDSC:
-
Myeloid-derived suppressor cell
- MHC:
-
Major histocompatibility complex
- NCI:
-
National Cancer Institute
- NK cell:
-
Natural killer cell
- PBMC:
-
Peripheral blood mononuclear cells
- PGE2:
-
Prostaglandin E2
- PHA:
-
Phytohaemagglutinin
- PI:
-
Proliferation index
- QLQ-C30:
-
Quality of life questionnaire-Core 30
- RBC:
-
Red blood cell
- SGOT:
-
Serum glutamic oxaloacetate transaminase
- SGPT:
-
Serum glutamic pyruvate transaminase
- TAA:
-
Tumor-associated antigen
- TAM:
-
Tumor-associated macrophage
- Tc:
-
T catalytic lymphocyte
- Th:
-
T helper lymphocyte
- TIL:
-
Tumor infiltrating lymphocytes
- TNFα:
-
Tumor necrosis factor-alpha
- WBC:
-
White blood cell
References
Van-den-Eynde BJ, Van-der-Bruggen P (1997) T cell defined tumor antigens. Curr Opin Immunol 9:684–693
Salgaller ML, Thurner M, Bartsch G, Boynton AL, Murphy GP (1999) Report from the International Union Agaist Cancer (UICC) Tumor Biology Committee: UICC Workshop on the use of dendritic cells in cancer clinical trials. Cancer 86:2674–2683
Sokolof MH, Vogelzang N (1999) The ongoing evolution of dendritic cell therapy. Cancer 86:2593–2596
Knuth A, Wölfel T, Klehmann E, Boon T, Büschenfelde KM (1989) Cytolytic T-cell clones against an autologous human melanoma: specificity study and definition of three antigens by immunoselection. Proc Natl Acad Sci U S A 86:2804–2808
Davis ID, Jefford M, Parente P, Cebon J (2003) Rational approaches to human cancer immunotherapy. J Leuk Biol 73:3–29
Liviu V, Titu E, John R, Monson E, Greenman J (2002) The role of CD8+ T cells in immune responses to colorectal cancer. Cancer Immunol Immunother 51:235–247
Rock KL, Gamble S, Rothstein L (1990) Presentation of exogenous antigen with class I major histocompatibility complex molecules. Science 249:918–921
Tatsumi T, Takehara T, Kanto T, Miyagi T, Kuzushita N, Sugimoto Y, Jinushi M, Kasahara A, Sasaki Y, Hori M, Hayashi N (2001) Adminstration of interleukin 12 enhances the therapeutic efficacy of dendritic cell-based tumor vaccines in mouse hepatocellular carcinoma. Cancer Res 61:7563–7567
Tuting T, Wilson CC, Martin DM, Kasamon YL, Rowles J, Ma DI, Slingluff CLJ, Wagner SN, Bruggen P, Baar J, Lotze MT, Storkus WJ (1998) Autologous human monocyte-derived dendritic cells genetically modified to express melanoma antigens elicit primary cytotoxic T cell responses in vitro: enhancement by co-transfection of genes encoding the Th1-biasing cytokines IL-12 and IFN alfa. J Immunol Methods 160:1139–1147
Shimizu K, Fields RC, Giedlin M, Mule JJ (1999) Systemic adminstration of interleukin 2 enhances the therapeutic effficacy of dendritic cell-based tumor vaccine. Proc Natl Acad Sci USA 96:2268–2273
Chen S, Akbar SM, Tanimoto K, Ninomiya T, Iuchi H, Michitaka K, Horiike N, Onji M (2000) Absence of CD83(+)-positive mature and activated dendritic cells at cancer nodules from patients with hepatocellular carcinoma: relevance to hepatocarcinogenesis. Cancer Lett 148:49–57
Van-de-Velde AL, Berneman ZN, Van-Tendeloo VF (2008) Immunotherapy of hematological malignancies using dendritic cells. Bull Cancer 95:320–326
Draube A, Klein-González N, Mattheus S, Brillant C, Hellmich M, Engert A, von Bergwelt-Baildon M (2011) Dendritic cell based tumor vaccination in prostate and renal cell cancer: a systematic review and meta-analysis. LoS One 6:e18801
Alexandrescu DT, Ichim TE, Riordan NH, Marincola FM, Di-Nardo A, Kabigting FD, Dasanu CA (2010) Immunotherapy for melanoma: current status and perspectives. J Immunother 33:570–590
Mazzolini G, Murillo O, Atorrasagasti C, Dubrot J, Tirapu I, Rizzo M, Arina A, Alfaro C, Azpilicueta A, Berasain C, Perez-Gracia JL, Gonzales A, Melero I (2007) Immunotherapy and immunoescape in colorectal cancer. World J Gastroenterol 13:5822–5831
Dauer M, Schnurr M, Eigler A (2008) Dendritic cell-based cancer vaccination: quo vadis? Expert Rev Vaccines 7:1041–1053
Nencioni A, Grünebach F, Schmidt SM, Müller MR, Boy D, Patrone F, Ballestrero A, Brossart P (2008) The use of dendritic cells in cancer immunotherapy. Crit Rev Oncol Hematol 65:191–199
Vulink A, Radford KJ, Melief C, Hart DN (2008) Dendritic cells in cancer immunotherapy. Adv Cancer Res 99:363–407
Erreni M, Mantovani A, Allavena P (2011) Tumor-associated Macrophages (TAM) and Inflammation in Colorectal Cancer. Cancer Microenviron 4:141–154
Roxburgh C, McMillan D (2010) Role of systemic inflammatory response in predicting survival in patients with primary operable cancer. Future Oncol 6:149–163
Roxburgh C, McMillan D (2012) The role of the in situ local inflammatory response in predicting recurrence and survival in patients with primary operable colorectal cancer. Cancer Treat Rev 38(5):451–466. doi:10.1016/j.ctrv.2011.09.001, Epub 2011 Sep 25
database-online http://www.cancer.org/Cancer/ColonandRectumCancer/DetailedGuide/colorectal-cancer-survival-rates. Last Medical Review: 05/24/2012; Last Revised: 01/17/2013
Palucka K, Banchereau J, Mellman I (2010) Designing vaccines based on biology of human dendritic cell subsets. Immunity 33:464–478
Nestle F, Banchereau J, Hart D (2001) Dendritic cells: on the move from bench to bedside. Nat Med 7:761–765
Figdor C, Jd V, Lesterhuis W, Melief C (2004) Dendritic cell immunotherapy: mapping the way. Nat Med 10:475–480
Dhodapkar MV, Krasovsky J, Steinman RM, Bhardwaj N (2000) Mature dendritic cells boost functionally superior CD8(+) T-cell in humans without foreign helper epitopes. J Clin Invest 105:9–14
Jonuleit H, Giesecke-Tuettenberg A, Tuting T, Thurner-Schuler B, Stuge TB, Paragnik L, Kandemir A, Lee PP, Schuler G, Knop J, Enk AH (2001) A comparison of two types of dendritic cells as adjuvants for the induction of melanoma-specific T-cell responses in humans following intranodal injection. Int J Cancer 93:243–251
Banchereau J, Palucka AK, Dhodapkar M, Burkeholder S, Taquet N, Rolland A, Taquet S, Coquery S, Wittkowski KM, Bhardwaj N (2001) Immune and clinical responses in patients with metastatic melanoma to CD34(+) progenitro-derived dendritic cell vaccine. Cancer Res 61:6451–6458
Schuler-Thurnmer B, Schultz ES, Berger TG, Weinlich G, Ebner S, Woerl P, Bender A, Feuerstein B, Fritsch PO, Romani N, Schuler G (2002) Rapid induction of tumor-specific type 1 T helper cells in metastatic melanoma patients by vaccination with mature cryopreserved, peptide-loaded monocyte-derived dendritic cells. J Exp Med 195:1279–1288
Martin-Fontecha A, Sebastiani S, Höpken U, Uguccioni M, Lipp M, Lanzavecchia A, Sallusto F (2003) Regulation of dendritic cell migration to the draining lymph node: impact on T lymphocyte traffic and priming. J Exp Med 198:615–621
Jefford M, Maraskovsky E, Cebon J, Davies ID (2001) The use of dendritic cells in cancer therapy. Lancet Oncol 2(6):343–353
Steinman RM, Dhodapkar M (2001) Active immunization against cancer with dendritic cells: the near future. Int J Cancer 94:459–473
Cerundolo V, Hermans I, Salio M (2004) Dendritic cells: a journey from laboratory to clinic. Nat Immunol 5:7–10
Cranmer L, Trevor K, Hersh E (2004) Clinical applications of dendritic cell vaccination in the treatment of cancer. Cancer Immunol Immunother 53:275–306
Kavanagh B, Ko A, Venook A, Margolin K, Zeh H, Lotze M, Schillinger B, Liu W, Lu Y, Mitsky P, Schilling M, Bercovici N, Loudovaris M, Guillermo R, Lee SM, Bender J, Mills B, Fong L (2007) Vaccination of metastatic colorectal cancer patients with matured dendritic cells loaded with multiple major histocompatibility complex class I peptides. J Immunother 30:762–772
Mocellin S, Rossi C, Lise M, Nitti D (2004) Colorectal cancer vaccines: principles, results, and perspectives. Gastroenterology 127:1821–1837
Mosolits S, Ullenhag G, Mellstedt H (2005) Therapeutic vaccination in patients with gastrointestinal malignancies. A review of immunological and clinical results. Ann Oncol 16:847–862
Nagorsen D, Thiel E (2006) Clinical and immunologic responses to active specific cancer vaccines in human colorectal cancer. Clin Cancer Res 12:3064–3069
Ridgway D (2003) The first 1000 dendritic cell vaccinees. Cancer Invest 21:873–886
Rosenberg S (2004) Development of effective immunotherapy for the treatment of patients with cancer. J Am Coll Surg 198:685–696
Rosenberg S, Yang J, Restifo N (2004) Cancer immunotherapy: moving beyond current vaccines. Nat Med 10:909–915
Lesterhuis WJ, De Vries IJ, Schreibelt G, Schuurhuis DH, Aarntzen EH, De Boer A, Scharenborg NM, Van De Rakt M, Hesselink EJ, Figdor CG, Adema GJ, Punt CJ (2010) Immunogenicity of dendritic cells pulsed with CEA peptide or transfected with CEA mRNA for vaccination of colorectal cancer patients. Anticancer Res 30:5091–5097
Okuno K, Sugiura F, Itoh K, Yoshida K, Tsunoda T, Nakamura Y (2012) Recent advances in active specific cancer vaccine treatment for colorectal cancer. Curr Pharm Biotechnol(Feb 14), PMID: 22339221
Sakakibara M, Kanto T, Hayakawa M, Kuroda S, Miyatake H, Itose I, Miyazaki M, Kakita N, Higashitani K, Matsubara T, Hiramatsu N, Kasahara A, Takehara T, Hayashi N (2011) Comprehensive immunological analyses of colorectal cancer patients in the phase I/II study of quickly matured dendritic cell vaccine pulsed with carcinoembryonic antigen peptide. Cancer Immunol Immunother 60:1565–1575
Zhou Q, Peng R, Wu X, Xia Q, Hou J, Ding Y, Zhou Q, Zhang X, Pang Z, Wan D, Zeng Y, Zhang X (2010) The density of macrophages in the invasive front is inversely correlated to liver metastasis in colon cancer. J Transl Med 8:13
Nagaraj S, Gabrilovich DI (2012) Regulation of suppressive function of myeloid-derived suppressor cells by CD4(+) T cells. Semin Cancer Biol 22(4):282–288. doi:10.1016/j.semcancer.2012.01.010, Epub 2012 Jan 31
Erdman S, Sohn J, Rao V, Nambiar P, Ge Z, Fox J, Schauer D (2005) CD4 + CD25+ regulatory lymphocytes induce regression of intestinal tumors in ApcMin/+ mice. Cancer Res 65:3998–4004
Chaput N, Louafi S, Bardier A, Charlotte F, Vaillant J, Ménégaux F, Rosenzwajg M, Lemoine F, Klatzmann D, Taieb J (2009) Identification of CD8 + CD25 + Foxp3+ suppressive T cells in colorectal cancer tissue. Gut 58:520–529
Nagorsen D, Voigt S, Berg E, Stein H, Thiel E, Loddenkemper C (2007) Tumor-infiltrating macrophages and dendritic cells in human colorectal cancer: relation to local regulatory T cells, systemic T-cell response against tumor-associated antigens and survival. J Transl Med 29:62
Gilboa E (2001) The risk of autoimmunity asssociated with tumor immunotherapy. Nat Immunol 2:789–792
Author information
Authors and Affiliations
Corresponding author
Additional information
János Hunyadi and Csilla András contributed equally to this work
Rights and permissions
About this article
Cite this article
Hunyadi, J., András, C., Szabó, I. et al. Autologous Dendritic Cell Based Adoptive Immunotherapy of Patients with Colorectal Cancer—A Phase I-II Study. Pathol. Oncol. Res. 20, 357–365 (2014). https://doi.org/10.1007/s12253-013-9704-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12253-013-9704-3