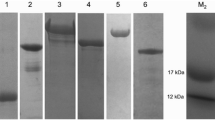
Abstract
Due to the recorded spreading of ticks in past years, a higher incidence of tick-borne diseases (TBDs) can be expected in the future in endemic areas, but can also pose an emerging public health concern in areas where they have not yet been recognized. Assessment of the exposure of vulnerable hosts to ticks would be a very helpful tool for TBD epidemiological studies, as well as for their proper managing. To confirm previous tick bites, the method of choice is detection of antibodies in host serum as markers developed against injected tick saliva proteins during feeding. We recently showed that the recombinant form of Ixodes ricinus AV422 saliva protein (rIrAV422) can serve for detection of markers in experimentally infested rats. Here we examine whether it can be used in the same manner in naturally exposed hosts. We chose hunting dogs as good sentinel animals. The study group consisted of 15 dogs that varied in breed, age, sex, previous tick infestation history and repellent treatment. Western blot analysis with rIrAV422 as an antigen confirmed the presence of tick bite markers in all analysed dogs. For some of the dogs, their previous tick infestation history was unclear, which emphasizes the usefulness of rIrAV422 for revealing it. Since hunting dogs are naturally infested with different ticks, the potential of rIrAV422 in assessment of general exposure to ticks is highlighted. Use of rIrAV422 can also be helpful in veterinary practice and research as a tool for validation of the efficiency of tick repellent products.

Similar content being viewed by others
References
Alarcon-Chaidez F, Ryan R, Wikel S, Dardick K, Lawler C, Foppa IM, Tomas P, Cushman A, Hsieh A, Spielman A, Bouchard KR, Dias F, Aslanzadeh J, Krause PJ (2006) Confirmation of tick bite by detection of antibody to Ixodes calreticulin salivary protein. Clin Vaccine Immunol 13:1217–1222. doi:10.1128/CVI.00201-06
Beugnet F (2002) Guide to major vector-borne diseases. Merial, S.A.S., Lyon
Coimbra-Dores MJ, Nunes T, Dias D, Rosa F (2016) Rhipicephalus sanguineus (Acari: Ixodidae) species complex: morphometric and ultrastructural analyses. Exp Appl Acarol 70:455–468. doi:10.1007/s10493-016-0095-5
Danielova V, Rudenko N, Daniel M, Holubova J, Materna J, Golovchenko M, Schwarzova L (2006) Extension of Ixodes ricinus ticks and agents of tick-borne diseases to mountain areas in the Czech Republic. Int J Med Microbiol 296(Suppl 40):48–53. doi:10.1016/j.ijmm.2006.02.007
Dennis DT, Piesman J (2005) Overview of tick-borne infections of humans. In: Goodman JL, Dennis DT, Sonenshine DE (eds) Tick-borne diseases of humans. American Society of Microbiology, Washington, pp 3–11
Földvári G, Farkas R (2005) Ixodid tick species attaching to dogs in Hungary. Vet Parasitol 129:125–131. doi:10.1016/j.vetpar.2004.11.032
Földvári G, Márialigeti M, Solymosi N, Lukács Z, Majoros G, Kósa JP, Farkas R (2007) Hard ticks infesting dogs in Hungary and their infection with Babesia and Borrelia species. Parasitol Res 101:25–34. doi:10.1007/s00436-007-0608-6
Fritz CL (2009) Emerging tick-borne diseases. Vet Clin N Am Small 39:265–278. doi:10.1016/j.cvsm.2008.10.019
Grisi L, Massard CL, Moya BGE, Pereira JB (2002) Impacto econômico das principais ectoparasitoses em bovinos no Brasil. A Hora Veterinária 21:8–10 (in Portuguese)
Guerrero FD, Nene VM, George JE, Barker SC, Willadsen P (2006) Sequencing a new target genome: the Boophilus microplus (Acari: Ixodidae) genome project. J Med Entomol 43:9–16. doi:10.1093/jmedent/43.1.9
Hornok S, Dénes B, Meli ML, Tánczos B, Fekete L, Gyuranecz M, de la Fuente J, Fernández de Mera IG, Farkas R, Hofmann-Lehmann R (2013) Non-pet dogs as sentinels and potential synanthropic reservoirs of tick-borne and zoonotic bacteria. Vet Microbiol 167:700–703. doi:10.1016/j.vetmic.2013.08.011
Jore S, Viljugrein H, Hofshagen M, Brun-Hansen H, Kristoffersen AB, Nygard K, Brun E, Ottesen P, Saevik BK, Ytrehus B (2011) Multi-source analysis reveals latitudinal and altitudinal shifts in range of Ixodes ricinus at its northern distribution limit. Parasite Vector 4:84. doi:10.1186/1756-3305-4-84
Kim TK, Radulović Ž, Mulenga A (2016a) Target validation of highly conserved Amblyomma americanum tick saliva serine protease inhibitor 19. Ticks Tick Borne Dis 7:405–414. doi:10.1016/j.ttbdis.2015.12.017
Kim TK, Tirloni L, Pinto AFM, Moresco J, Yates JR III, Vaz IdS, Mulenga A Jr (2016b) Ixodes scapularis tick saliva proteins sequentially secreted every 24 h during blood feeding. PLoS Negl Trop Dis. doi:10.1371/journal.pntd.0004323
Korch GW Jr (1994) Geographic dissemination of tick-borne zoonoses. In: Sonenshine DE, Mather TN (eds) Ecological dynamics of tick-borne zoonoses. Oxford University Press, New York, pp 139–197
Lewis L, Radulović Ž, Kim TK, Porter LM, Mulenga A (2015) Identification of 24 h Ixodes scapularis tick saliva proteins. Ticks Tick Borne Dis 6:424–434. doi:10.1016/j.ttbdis.2015.03.012
Lim S, Irwin PJ, Lee SR, Oh MH, Ahn KS, Myung BY, Shin SS (2010) Comparison of selected canine vector-borne diseases between urban animal shelter and rural hunting dogs in Korea. Parasite Vector 3:32. doi:10.1186/1756-3305-3-32
Mihaljica D, Marković D, Radulović Ž, Mulenga A, Ćakić S, Sukara R, Samardžić J, Tomanović S (2017) Ixodes ricinus immunogenic saliva protein, homologue to Amblyomma americanum AV422: determining its potential for use in tick bite confirmation. Ticks Tick Borne Dis 8:391–395. doi:10.1016/j.ttbdis.2017.01.001
Milutinović M, Radulović Ž (2002) Ecological notes on ticks (Acari: Ixodidae) in Serbia (Central regions). Acta Vet Beograd 52:49–57. doi:10.2298/AVB0201049M
Milutinović M, Aleksić-Barkač N, Pavlović I (1998) Faunistic and ecological notes on ticks (Acari: Ixodidae, Argasidae) in the extended area of Belgrade. Magy Allatorvosok 120:434–436
Mulenga A, Blandon M, Khumthong R (2007) The molecular basis of the Amblyomma americanum tick attachment phase. Exp Appl Acarol 41:267–287. doi:10.1007/s10493-007-9064-3
Mulenga A, Kim TK, Ibelli AMG (2013) Deorphanization and target validation of cross-tick species conserved novel Amblyomma americanum tick saliva protein. Int J Parasitol 43:439–451. doi:10.1016/j.ijpara.2012.12.012
Paddock CD, Telford SR III (2011) Through a glass, darkly: the global incidence of tick-borne diseases. In: Critical needs and gaps in understanding prevention, amelioration, and resolution of lyme and other tick-borne diseases: the short-term and long-term outcomes. Workshop report. National Academies Press, Washington, DC, p 37
Parola P, Raoult D (2001) Ticks and tick-borne bacterial diseases in humans: an emerging infectious threat. Clin Infect Dis 32:897–928. doi:10.1086/319347
Pérez Vera C, Kapiainen S, Junnikkala S, Aaltonen K, Spillmann T, Vapalahti O (2014) Survey of selected tick-borne diseases in dogs in Finland. Parasite Vector 7:285. doi:10.1186/1756-3305-7-285
Potkonjak A, Gutiérrez R, Savić S, Vračar V, Nachum-Biala Y, Jurišić A, Kleinerman G, Rojas A, Petrović A, Baneth G, Harrus S (2016) Molecular detection of emerging tick-borne pathogens in Vojvodina, Serbia. Ticks Tick Borne Dis 7:199–203. doi:10.1016/j.ttbdis.2015.10.007
Radulović ŽM, Kim TK, Porter LM, Sze SH, Lewis L, Mulenga A (2014) A 24–48 h fed Amblyomma americanum tick saliva immune-proteome. BMC Genom 15:518. doi:10.1186/1471-2164-15-518
Rajput Z, Hu S, Chen WJ, Arijo AG, Xiao CW (2006) Importance of ticks and their chemical and immunological control in livestock. J Zhejiang Univ Sci B 7:912–921. doi:10.1631/jzus.2006.B0912
Shaw SE, Day MJ, Birtles RJ, Breitschwerdt EB (2001) Tick-borne infectious diseases of dogs. Trends Parasitol 17:74–80. doi:10.1016/S1471-4922(00)01856-0
Tirloni L, Reck J, Terra RM, Martins JR, Mulenga A, Sherman NE, Fox JW, Yates JR III, Termignoni C, Pinto AFM, Vaz IDS Jr (2014) Proteomic analysis of cattle tick Rhipicephalus (Boophilus) microplus saliva: a comparison between partially and fully engorged females. PLoS ONE 9(4):e94831. doi:10.1371/journal.pone.0094831
Tirloni L, Islam MS, Kim TK, Diedrich JK, Yates JR III, Pinto AFM, Mulenga A, You MJ, Vaz IDS Jr (2015) Saliva from nymph and adult females of Haemaphysalis longicornis: a proteomic study. Parasite Vector 8:338. doi:10.1186/s13071-015-0918-y
Vu Hai V, Almeras L, Socolovschi C, Raoult D, Parola P, Pagès F (2014) Monitoring human tick-borne disease risk and tick bite exposure in Europe: available tools and promising future methods. Ticks Tick Borne Dis 5:607–619. doi:10.1016/j.ttbdis.2014.07.022
Zygner W, Gorski P, Wedrychowicz H (2009) New localities of Dermacentor reticulatus tick (vector of Babesia canis canis) in central and eastern Poland. Pol J Vet Sci 12:549–555. doi:10.1016/j.ttbdis.2015.05.007
Acknowledgements
This work was supported by a grant from the Ministry of Education, Science, and Technological Development, Republic of Serbia (Project No. 173006). The authors would like to thank Prof. Milica Kovačević-Filipović (FVM, Belgrade) for valuable help in interpreting the results.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The protocol was approved by the Ministry of Agriculture and Environmental Protection, Republic of Serbia (permission number 323-07-03455/2015-05/3), which is in accordance with the National Law on Animal Welfare and consistent with guidelines for animal research and principles recommended by the Directive on the Protection of Animals Used for Scientific Purposes (Directive 2010/63/EU of the European Parliament and Council, 22 September 2010).
Rights and permissions
About this article
Cite this article
Mihaljica, D., Marković, D., Radulović, Ž. et al. Assessment of using recombinant Ixodes ricinus AV422 saliva protein for confirmation of tick bites in hunting dogs as naturally infested hosts. Exp Appl Acarol 72, 429–437 (2017). https://doi.org/10.1007/s10493-017-0170-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10493-017-0170-6