Abstract
Introduction:
This study aims to determine the urine concentrations of malondialdehyde (MDA), and tumor necrosis factor-alpha (TNF-α) biomarkers with pain intensity in a patient with low back pain (LBP) compared to healthy controls. Also, the correlation between these biomarkers and clinical findings of pain intensity was determined during 25 weeks to find a molecular diagnostic factor of disease progression.Case Presentation:
Participants in this case study are students at Shahed University, Iran. A 22‑year‑old man patient with a three-month history of progressive LBH and gait instability and an age, sex, and body mass index (BMI) matched healthy man was enrolled for 25 weeks. Spinal MRI (Siemens 1.5 T) revealed disc degeneration and displacement. LBP symptoms were diagnosed by a neurologist using ICD-9 diagnostic codes. Other diseases that affect the urine levels of MDA and TNF-α in the patient, such as allergic or infectious diseases, have been ruled out. After collecting demographic information, the facial pain scale (FPS) and numerical rating scale (NRS) for pain and 24-hour urine samples of a healthy person and a patient were collected weekly for 25 weeks. Samples were analyzed for MDA and TNF-α.Conclusions:
During the experiment, urinary MDA levels were significantly elevated while TNF-α levels were not changed in LBP patients compared to control individuals (P ≤ 0.001). Furthermore, the assessment of LBP by the FPS and NRS pain scores was only correlated with urinary MDA levels, and urinary TNF-α levels did not change during the experiment. Pearson correlation analysis revealed a positive correlation between pain scores during 25 weeks and urine concentrations of MDA (r = 0.60, P-value < 0.001). According to the present study, chronic LBP patients had higher levels of MDA in their urine than those who did not have chronic LBP. There was a significant positive association between urine MDA level and pain intensity progression.Keywords
Back Pain Tumor Necrosis Factor-Alpha Malondialdehyde Oxidative Stress
1. Introduction
Nearly 80% of people around the world have experienced low back pain (LBP) at least once in their lifetime (1). It is categorized into acute, sub-acute, and chronic. There is a very high transition rate between acute and chronic low back pain due to the lack of accurate diagnostic symptoms, and chronic low back pain patients experience recurrent low back pain throughout their lives (2). Although many efforts have been made to determine the cause of the progression of low back pain, its exact pathophysiologic mechanism remains poorly understood. Multiple factors have been implicated in the pathogenesis of LBP, including elevated levels of oxidative stress and inflammatory factors (3, 4). Currently, no accurate diagnostic biomarkers have been identified for the progression from acute to chronic low back pain. Increasing evidence suggests that oxidative stress plays a role in low back pain and the possibility of changing from an acute to a chronic condition in patients suffering from intervertebral disc degeneration (5). It is necessary, however, to examine more clinical presentations, especially inflammation and oxidative stress factors in relation to LBP, in order to establish their potential role in the diagnosis and treatment of this disease. The need for further research into the factors involved in the transformation of acute to chronic conditions of this disease has been highlighted by researchers (6). The primary aim of this case study was to compare urine concentrations of malondialdehyde (MDA) and tumor necrosis factor-alpha (TNF-α) between a patient and age, sex, and body mass index (BMI) matched healthy control. The secondary aim of this study was to determine the correlation between these biomarkers with pain progression after the caseation of analgesic therapy.
2. Case Presentation
The patient was a 22-year-old man with moderate LBP who presented to a specialist with a three-month history of progressive backache and difficulty maintaining correct posture. There was no history of physical trauma around the onset of symptoms in the patient. He did not have any other medical or familial history. A routine blood biochemistry test and a hematological test revealed no abnormalities. Physical examinations and MRIs revealed anomalies in the lumbosacral area involving the L1 - S1 vertebral disks. Images and MRIs showed lumbar disc degeneration from the L4 - L5 and L5 - S1 discs to be evenly distributed among the four lowermost discs. The straight leg raising (SLR) test has been used as the primary test to diagnose lumbar disc herniation. The International Classification of Diseases (ICD-9-CM) diagnosis codes were applied to patients with mechanical low back problems. The researcher measured patient pain intensity using the Facial Pain Scale (FPS) and Numerical Rating Scale (NRS), and pain scores were correlated with urine biomarkers. Urinary MDA and TNF-α levels were measured together with other serological tests. He had no other complaint which affected his serum lipid peroxidation product levels. An age- and gender-matched healthy person was used as a control. During the 25-week sampling period, 24-hour urine samples of the patient and healthy person were collected weekly, and the commercial assay kit measured urinary MDA and TNF-α.
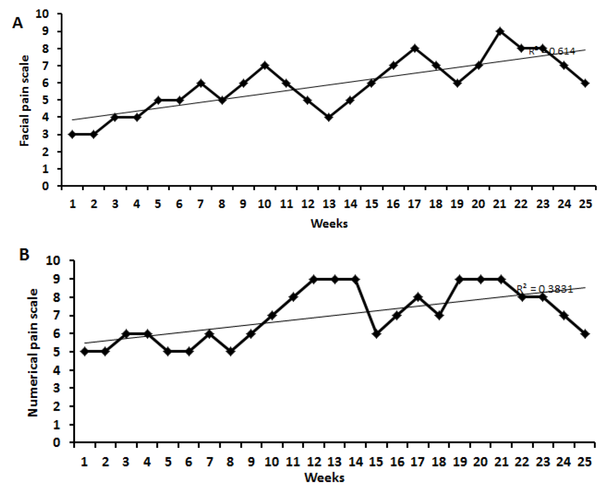
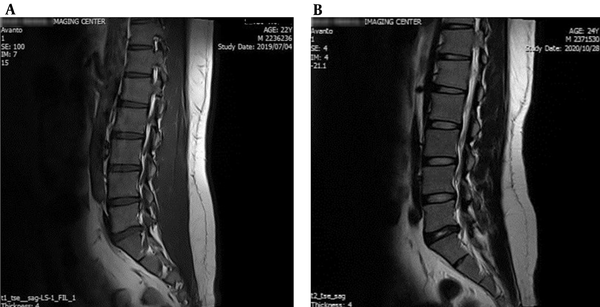
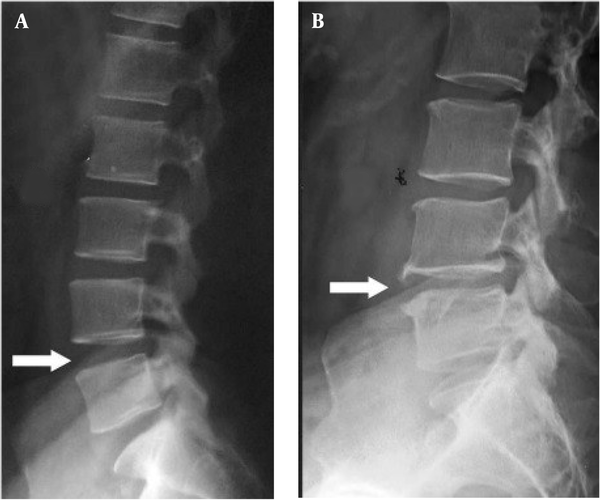
In Figure 1A and B, patients preferred FPS (n = 3 to 9) and NRS (n = 5 to 9). The result of the SLR test in the patient was significantly (P < 0.01) different compared to the healthy control. The MRI imaging revealed degeneration and herniation of the lumbar disk in the L5 - S1 region with nerve root compression and disc prolapse, confirmed by the radiologist (Figure 2A and B). The conus medullaris and nerve roots presented normal signal intensity and no signs of compression or pathological enhancement. X-ray studies of the lumbar spine showed little instability, and disc height and alignment were abnormal (Figure 3A and B). Compared with healthy subjects, the patient with LBP had significantly higher mean concentrations of MDA but no difference in TNF-α. During the experiment, urinary MDA concentrations gradually increased (P < 0.01) without any significant (P < 0.01) change in urinary TNF-α levels compared to a healthy subject (Figure 4A and B). In the patient, higher MDA concentrations in urine were associated with higher pain intensity scores (Figure 4C).
Numeric rating scale (NRS) and facial pain scale (FPS) rating. A, Changes in NRS; and B, FPS rates in the low back pain patient during the experimental period.
The MRI images from the lumbosacral spinal region. A, The sagittal T1WI show degenerative changes as disk dehydration and inner nuclear cleft noted at all lumbar spine; B, Disk dehydration and a left paracentral disk protrusion were observed at L3/L4 and L5/S1 levels. The conus medullaris is normal at L1 (the imaged soft tissue was interpreted as normal).
X-ray films of the lumbar spine from A, Lateral view; and B, Lateral view with extension. These X-ray films show instability and abnormal disc height and alignment. Plain X-rays of the lumbar spine (A) show a slight loss of height with anterior wedging of L2.
A, Change of malondialdehyde (MDA); and B, Tumor necrosis factor-alpha (TNF-α) in the urine of the LBP patient in comparison with the healthy person during 25 weeks; C, Correlation of numeric pain scores and urinary MDA levels in the LBP patient for 25 weeks.

3. Discussion
The current study found that urinary levels of MDA, a product of lipid oxidation, were higher in patients with LBP compared to healthy subjects, but TNF-α, a biomarker of systemic inflammation, showed no difference. Although serum concentrations of inflammatory indices have been researched in patients with painful intervertebral disc degeneration, data regarding urine concentrations of MDA and TNF-α in LBP patients are limited (7). Clinical studies have shown that low back and musculoskeletal pain improvements were accompanied by a significant reduction in MDA serum levels (8).
There is a concurrent increase in serum MDA levels in patients with lumbar disc degeneration disease, as well as a significant reduction (P < 0.01) in serum endogenous antioxidant levels (9). Similar to our finding in this study, other researchers did not find any significant differences in TNF-α (serum or CSF) concentrations between patients with different pain syndromes (10).
In addition, a previous report indicated that serum lipid peroxidation factors were associated with pain and limitations in joint mobility in patients (11). There was a significant (P < 0.01) relationship between pain score and blood MDA level in premature neonates after having been exposed to painful stimuli in another case study (12). Additionally, no differences were detected in the inflammatory biomarkers in the serum of individuals with chronic low back pain (13). Based on studies in animals and humans, elevated reactive oxygen species (ROS) levels are related to the release of substance-P at the spinal level, which is a critical neurotransmitter peptide involved in promoting pain transmission in the intervertebral discs (14). Also, it has been reported that serum MDA levels are significantly higher in workers suffering from work-related musculoskeletal pain or impairment in the lower back or upper extremities (15). In this study, the increase in urinary excretion of MDA in patients with LBP could explain the gradual increase in pain caused by the increase in general levels of MDA in the body. In conclusion, we sought to draw attention to the slow progression of LBP associated with systemic lipid peroxidation and increased urinary MDA levels. As such, it seems that MDA may be a convenient, accessible, and possibly useful biomarker for diagnosing the progression of LBP. The current findings further support the relationship between oxidative stress and LBP pathophysiology. Thus, lipid peroxidation management seems promising for preventing LBP progress.
Acknowledgements
References
-
1.
Meucci RD, Fassa AG, Faria NM. Prevalence of chronic low back pain: systematic review. Rev Saude Publica. 2015;49. [PubMed ID: 26487293]. [PubMed Central ID: PMC4603263]. https://doi.org/10.1590/S0034-8910.2015049005874.
-
2.
Stevans JM, Delitto A, Khoja SS, Patterson CG, Smith CN, Schneider MJ, et al. Risk Factors Associated With Transition From Acute to Chronic Low Back Pain in US Patients Seeking Primary Care. JAMA Netw Open. 2021;4(2). e2037371. [PubMed ID: 33591367]. [PubMed Central ID: PMC7887659]. https://doi.org/10.1001/jamanetworkopen.2020.37371.
-
3.
Kolberg C, Horst A, Moraes MS, Duarte FC, Riffel AP, Scheid T, et al. Peripheral oxidative stress blood markers in patients with chronic back or neck pain treated with high-velocity, low-amplitude manipulation. J Manipulative Physiol Ther. 2015;38(2):119-29. [PubMed ID: 25487299]. https://doi.org/10.1016/j.jmpt.2014.11.003.
-
4.
Teodorczyk-Injeyan JA, Triano JJ, Injeyan HS. Nonspecific Low Back Pain: Inflammatory Profiles of Patients With Acute and Chronic Pain. Clin J Pain. 2019;35(10):818-25. [PubMed ID: 31283548]. [PubMed Central ID: PMC6735949]. https://doi.org/10.1097/AJP.0000000000000745.
-
5.
Cao G, Yang S, Cao J, Tan Z, Wu L, Dong F, et al. The Role of Oxidative Stress in Intervertebral Disc Degeneration. Oxid Med Cell Longev. 2022;2022:2166817. [PubMed ID: 35069969]. [PubMed Central ID: PMC8769842]. https://doi.org/10.1155/2022/2166817.
-
6.
Hartvigsen J, Hancock MJ, Kongsted A, Louw Q, Ferreira ML, Genevay S, et al. What low back pain is and why we need to pay attention. Lancet. 2018;391(10137):2356-67. [PubMed ID: 29573870]. https://doi.org/10.1016/S0140-6736(18)30480-X.
-
7.
Lyu FJ, Cui H, Pan H, Mc Cheung K, Cao X, Iatridis JC, et al. Painful intervertebral disc degeneration and inflammation: from laboratory evidence to clinical interventions. Bone Res. 2021;9(1):7. [PubMed ID: 33514693]. [PubMed Central ID: PMC7846842]. https://doi.org/10.1038/s41413-020-00125-x.
-
8.
Siems W, Bresgen N, Brenke R, Siems R, Kitzing M, Harting H, et al. Pain and mobility improvement and MDA plasma levels in degenerative osteoarthritis, low back pain, and rheumatoid arthritis after infrared A-irradiation. Acta Biochim Pol. 2010;57(3):313-9. [PubMed ID: 20827448].
-
9.
Bakirezer SD, Yaltirik CK, Kaya AH, Yilmaz SG, Ozdogan S, Billur D, et al. The Evaluation of Glutathione Reductase and Malondialdehyde Levels in Patients With Lumbar Disc Degeneration Disease. In Vivo. 2019;33(3):811-4. [PubMed ID: 31028201]. [PubMed Central ID: PMC6559890]. https://doi.org/10.21873/invivo.11543.
-
10.
Ludwig J, Binder A, Steinmann J, Wasner G, Baron R. Cytokine expression in serum and cerebrospinal fluid in non-inflammatory polyneuropathies. J Neurol Neurosurg Psychiatry. 2008;79(11):1268-73. [PubMed ID: 18550631]. https://doi.org/10.1136/jnnp.2007.134528.
-
11.
Jensen GS, Ager DM, Redman KA, Mitzner MA, Benson KF, Schauss AG. Pain reduction and improvement in range of motion after daily consumption of an acai (Euterpe oleracea Mart.) pulp-fortified polyphenolic-rich fruit and berry juice blend. J Med Food. 2011;14(7-8):702-11. [PubMed ID: 21470042]. [PubMed Central ID: PMC3133683]. https://doi.org/10.1089/jmf.2010.0150.
-
12.
Slater L, Asmerom Y, Boskovic DS, Bahjri K, Plank MS, Angeles KR, et al. Procedural pain and oxidative stress in premature neonates. J Pain. 2012;13(6):590-7. [PubMed ID: 22543043]. [PubMed Central ID: PMC3367033]. https://doi.org/10.1016/j.jpain.2012.03.010.
-
13.
Boisson M, Borderie D, Henrotin Y, Teboul-Core S, Lefevre-Colau MM, Rannou F, et al. Serum biomarkers in people with chronic low back pain and Modic 1 changes: a case-control study. Sci Rep. 2019;9(1):10005. [PubMed ID: 31292506]. [PubMed Central ID: PMC6620434]. https://doi.org/10.1038/s41598-019-46508-x.
-
14.
Zheng J, Zhang J, Zhang X, Guo Z, Wu W, Chen Z, et al. Reactive Oxygen Species Mediate Low Back Pain by Upregulating Substance P in Intervertebral Disc Degeneration. Oxid Med Cell Longev. 2021;2021:6681815. [PubMed ID: 34093962]. [PubMed Central ID: PMC8140854]. https://doi.org/10.1155/2021/6681815.
-
15.
Ghosh S, Acharyya M, Majumder T, Bagchi A. Metabolic Signatures of Oxidative Stress and Their Relationship with Erythrocyte Membrane Surface Roughness Among Workers of Manual Materials Handling (MMH). N Am J Med Sci. 2015;7(12):558-66. [PubMed ID: 26942132]. [PubMed Central ID: PMC4755081]. https://doi.org/10.4103/1947-2714.172846.