Abstract
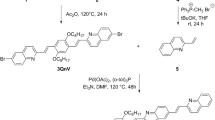
An oligomer (3) containing flexible hydrophilic hexa(ethylene glycol) and hydrophobic naphthalene-bisimide chromophores has been synthesized by a one-step condensation reaction and its photophysical and electrochemical properties were investigated. 3 was characterized through the data from NMR, IR, UV-vis, GPC, DSC, TGA, elemental analysis and cyclic voltammetry. The average molecular weight (Mw) of 3 was 4430 g mol−1. Intrinsic viscosity was measured as 0.28 dL g−1 in m-cresol at 25 °C. It has high thermal stability (Td = 325 °C). Interestingly, compound 3 shows excimer-like emission in all kinds of solvents. The band gap energy (Eg), LUMO and HOMO energy values in nonpolar and polar protic solvents were 2.71 eV/3.12 eV, −3.69 eV/−3.88 eV and −6.40 eV/−7.00 eV for 3, respectively. The oligomer showed concentration and solvent dependent fluorescent color tunability. Remarkably, the fluorescent colors of the excimer emissions at 10−6 M concentration in CHCl3, DMF and MeOH are light yellow, light blue-yellow and strong blue, respectively, and become more intense at higher concentrations. The excimer emission color in CHCl3 and DMF is fluorescent yellow and changed to green in MeOH at10−4 M concentration. 3 shows two reversible reduction steps at −1.103 and −1.457 V (vs. ferrocene/ferrocenium) in nonpolar solvent CH2Cl2 and only one at −0.917 V in (50: 50) CH3OH-CH3CN binary solvent mixture with higher reversibility. Strong blue-shifts of emission band were noted in protic solvents, which confirm the existence of a negative solvatochromism probably due to protonation. The strong solvent-dependent photophysical and electrochemical properties, including the large shift of excimer emission maximum reflecting self-assembly mediated through hydrogen bonding and π-stacking interactions, make the oligomer a potential candidate for various photo-sensing applications.
Similar content being viewed by others
References
C. Michot, D. Baril and M. Armand, Polyimide-polyether mixed conductors as switchable materials for electrochromic devices, Sol. Energy Mater. Sol. Cells, 1995, 39, 289–299.
H. Icil and S. Icli, Synthesis and properties of a new pho-tostable polymer: Perylene-3,4,9,10-tetracarboxylic acid-bis-(N,N′-dodecylpolyimide), J. Polym. Sci., Part A: Polym. Chem., 1997, 35, 2137–2142.
M. S. Cubberley and B. L. Iverson, 1H NMR investigation of solvent effects in aromatic stacking interactions, J. Am. Chem. Soc., 2001, 123, 7560–7563.
Y. Yin, O. Yamada, K. Tanaka and K. I. Okamoto, On the development of naphthalene-based sulfonated polyimide membranes for fuel cell applications, Polym. J., 2006, 38, 197–219.
K. Yuney and H. Icil, Synthesis, photochemical, and electrochemical properties of naphthalene-1,4,5,8-tetracarboxylic acid-bis-(N, N′-bis-(2,2,4(2,4,4)-trimethylhexylpolyimide)) and poly(N, N′-bis-(2,2,4(2,4,4)-trimethyl-6-aminohexyl)3,4,9,10-peryl-enetetracarboxdiimide), Eur. Polym. J., 2007, 43, 2308–2320.
J. B. Bodapati and H. Icil, Highly soluble perylene diimide and oligomeric diimide dyes combining perylene and hexa(ethylene glycol) units: Synthesis, characterization, optical and electrochemical properties, Dyes Pigm., 2008, 79, 224–235.
B. Jancy and S. K. Asha, Synthesis and self-organization properties of copolyurethanes based on perylenediimide and naphthalenediimide units, J. Polym. Sci., Part A: Polym. Chem., 2009, 47, 1224–1235.
S. Burattini, H. M. Colquhoun, J. D. Fox, D. Friedmann, B. W. Greenland, P. J. F. Harris, W. Hayes, M. E. Mackay and S. J. Rowan, A Self-repairing, supramolecular polymer system: healability as a consequence of donor-acceptor π-π stacking interactions, Chem. Commun., 2009, 6717–6719.
Y. Morisaki, J. A. Fernandes and Y. Chujo, Naphthalene-based oligothiophene-stacked polymers, Polym. J., 2010, 42, 928–934.
C. A. V. Walree, V. E. M. Kaats-Richters, L. W. Jenneskens, R. M. Williams and I. H. M. V. Stokkum, Intramolecular excimer and exciplex emission of 1,4-dipyrenyl substituted cyclohexasilane, Chem. Phys. Lett., 2002, 355, 65–70.
L. H. Gade, C. H. Galka, R. M. Williams, L. D. Cola, M. McPartlin, B. Dong and L. Chi, Synthesis, photophysical properties, and nanocrystal formation of a new class of tetra-n-substituted perylenes, Angew. Chem., Int. Ed., 2003, 42, 2677–2681.
B. Dong, C. H. Galka, L. H. Gade, L. Chi and R. M. Williams, Hydrogen-bond assisted formation of rod shaped organic nanocrys-tals: Control of the aggregational state and structural elucidation, Nanopages, 2006, 1, 325–328.
D. Veldman, S. M. A. Chopin, S. C. J. Meskers, M. M. Groeneveld, R. M. Williams and R. A. J. Janssen, Triplet formation involving a polar transition state in a well-defined intramolecular perylenediimide dimeric aggregate, J. Phys. Chem. A, 2008, 112, 5846–5857.
C. Hippius, I. H. M. V. Stokkum, E. Zangrando, R. M. Williams, M. Wykes, D. Beljonne and F. Würthner, Ground- and Excited-state pinched cone equilibria in calix[4]arenes bearing two perylene bisimide dyes, J. Phys. Chem. C, 2008, 112, 14626–14638.
C. Thalacker, C. Röger and F. Würthner, Synthesis and optical and redox properties of core-substituted naphthalene diimide dyes, J. Org. Chem., 2006, 71, 8098–8105.
C. Röger, M. G. Müller, M. Lysetska, Y. Miloslavina, A. R. Holzwarth and F. Würthner, Efficient energy transfer from peripheral chro-mophores to the self-assembled zinc chlorin rod antenna: a bioinspired light-harvesting system to bridge the “green gap”, J. Am. Chem. Soc., 2006, 128, 6542–6543.
C. Röger and F. Würthner, Core-tetrasubstituted naphthalene diimides: Synthesis, optical properties, and redox characteristics, J. Org. Chem., 2007, 72, 8070–8075.
C. Röger, Y. Miloslavina, D. Brunner, A. R. Holzwarth and F. Würthner, Self-assembled zinc chlorin rod antennae powered by peripheral light-harvesting chromophores, J. Am. Chem. Soc., 2008, 130, 5929–5939.
E. S-. Balcerzak, A. Iwan, M. Krompiec, M. Siwy, D. Tapa, A. Sikora and M. Palewicz, New thermotropic azomethine-naphthalene diimides for optoelectronic applications, Synth. Met., 2010, 160, 2208–2218.
S. V. Bhosale, C. H. Jani and S. J. Langford, Chemistry of naphthalene diimides, Chem. Soc. Rev., 2008, 37, 331–342.
J. G. Hansen, N. Feeder, D. G. Hamilton, M. J. Gunter, J. Becher and J. K. M. Sanders, Macrocyclization and molecular interlocking via mitsunobu alkylation: Highlighting the role of C-H ··· O interactions in templating, Org. Lett., 2000, 2, 449–452.
X. Z. Wang, X. Q. Li, X. B. Shao, X. Zhao, P. Deng, X. K. Jiang, Z. T. Li and Y. Q. Chen, Selective rearrangements of quadruply hydrogen-bonded dimer driven by donor-acceptor interaction, Chem.-Eur. J., 2003, 9, 2904–2913.
V. Steullet and D. W. Dixon, Self-stacking of naphthalene bis(dicarboximide)s probed by NMR, J. Chem. Soc., Perkin Trans. 2, 1999, 1547–1558.
X. Q. Li, D. J. Feng, X. K. Jiang and Z. T. Li, Donor-acceptor interaction-mediated arrangement of hydrogen bonded dimers, Tetrahedron, 2004, 60, 8275–8284.
O. Johansson, H. Wolpher, M. Borgström, L. Hammarström, J. Bergquist, L. Sun and B. Akermark, Intramolecular charge separation in a hydrogen bonded tyrosine-ruthenium(II)-naphthalene diimide triad, Chem. Commun., 2004, 194–195.
M. Tomasulo, D. M. Naistat, A. J. P. White, D. J. Williams and F. M. Raymo, Self-assembly of naphthalene diimides into cylindrical microstructures, Tetrahedron Lett., 2005, 46, 5695–5698.
Y. Ofir, A. Zelichenok and S. Yitzchaik, 1,4;5,8-naphthalene-tetracarboxylic diimide derivatives as model compounds for molecular layer epitaxy, J. Mater. Chem., 2006, 16, 2142–2149.
Z. Merican, K. D. Johnstone and M. J. Gunter, Porphyrin-naphthodiimide interactions as a structural motif in foldamers and supramolecular assemblies, Org. Biomol. Chem., 2008, 6, 2534–2543.
G. Koshkakaryan, L. M. Klivansky, D. Cao, M. Snauko, S. J. Teat, J. O. Struppe and Y. Liu, Alternative donor-acceptor stacks from crown ethers and naphthalene diimide derivatives: Rapid, selective formation from solution and solid state grinding, J. Am. Chem. Soc., 2009, 131, 2078–2079.
S. V. Bhosale, C. Jani, C. H. Lalander and S. J. Langford, Solvophobic control of core-substituted naphthalene diimide nanostructures, Chem. Commun., 2010, 46, 973–975.
H. Shao and J. R. Parquette, A p-conjugated hydrogel based on an fmoc-dipeptide naphthalene diimide semiconductor, Chem. Commun., 2010, 46, 4285–4287.
P. M. Alvey, J. J. Reczek, V. Lynch and B. L. Iverson, A systematic study of thermochromic aromatic donor-acceptor materials, J. Org. Chem., 2010, 75, 7682–7690.
A. J. Zych and B. L. Iverson, Synthesis and conformational characterization of tethered, self-complexing 1,5-dialkoxynaphthalene/1,4,5,8-naphthalenetetracarboxylic diimide systems, J. Am. Chem. Soc., 2000, 122, 8898–8909.
S. G. Ramkumar and S. Ramakrishnan, Understanding the folding process of synthetic polymers by small-molecule folding agents, J. Chem. Sci., 2008, 120, 187–194.
N. J. Turro, Molecular Photochemistry, ed. New York, W. A. Benjamin, Inc., 1965, pp. 4–48.
Z. Peng, Z. Bao and M. E. Galvin, Polymers with bipolar carrier transport abilities for light emitting diodes, Chem. Mater., 1998, 10, 2086–2090.
J. L. Bredas, R. Silbey, D. S. Boudreaux and R. R. Chance, Chain-length dependence of electronic and electrochemical properties of conjugated systems: polyacetylene, polyphenylene, polythiophene, and polypyrrole, J. Am. Chem. Soc., 1983, 105, 6555–6559.
A. J. Bard and L. R. Faulkner, Electrochemical methods, fundamentals and applications, New York, Wiley & Sons Inc., 1980.
S. Asir, A. S. Demir and H. Icil, The synthesis of novel, unsymmetrically substituted, chiral naphthalene and perylene diimides: Photophysical, electrochemical, chiroptical and intramolecular charge transfer properties, Dyes Pigm., 2010, 84, 1–13.
Author information
Authors and Affiliations
Corresponding author
Additional information
Electronic supplementary information (ESI) available. See DOI: 10.1039/c1pp05019b
Rights and permissions
About this article
Cite this article
Bodapati, J.B., Icil, H. A new tunable light-emitting and π-stacked hexa-ethyleneglycol naphthalene-bisimide oligomer: synthesis, photophysics and electrochemical properties. Photochem Photobiol Sci 10, 1283–1293 (2011). https://doi.org/10.1039/c1pp05019b
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1039/c1pp05019b